Reaction Network
A reaction network for catalytic reforming is shown in Figure 8.3 [2], indicating the role of metallic (M) and the acidic (A) sites on the support in catalyzing the chemical reactions. The surfaces of metals (e.g., Pt) catalyze dehydrogenation reactions, whereas the acid sites on the support (e.g., alumina) catalyze isomerization and cracking reactions. Metal and acid sites are involved in the catalysis of hydrocracking reactions. Achieving the principal objective of catalytic reforming—high yields and high quality of reformate—can be achieved, to a large extent, by controlling the activity of the catalysts and the balance between acidic and metallic sites to increase the selectivity to desirable reactions in the reaction network.
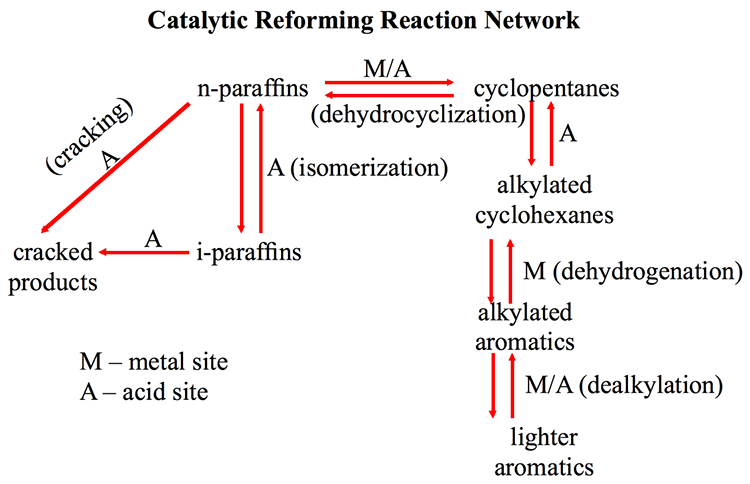
Catalytic Reforming Reaction network
KEY: R= Reversible, M = Metal Site, A=Acid Site
Lighter aromatics become alkylated aromatics (R)
-M/A (dealkylation)
Alkylated Aromatics become alkylated cyclohexanes (R)
-M (dehydrogenation)
Alkylated cyclohexanes become cyclopentanes (R)
-A
Cyclopentanes become n-paraffins (R)
-M/A (dehydrocyclization)
n-paraffins become i-paraffins (R)
-A (isomerization)
i-paraffins and n-paraffins to cracked products
-A (cracking)