As you've recently read, there are four principal bonding types: ionic, covalent, metallic, and van der Waals. Ionic bonding involves the exchange of electrons between atoms to complete shells, either by adding or giving up electrons. The resulting atoms are oppositely charged and attract each other, resulting in an ionic bond. Covalently bonded materials have bonds in which electrons are shared between atoms. In metallic bonding, a "sea of electrons" is uniformly distributed throughout the solid and acts as a glue to hold the atoms together. Van der Waals bonds are relatively weak compared to the other three principal bond types and result when attractive forces from permanent or induced dipoles form.
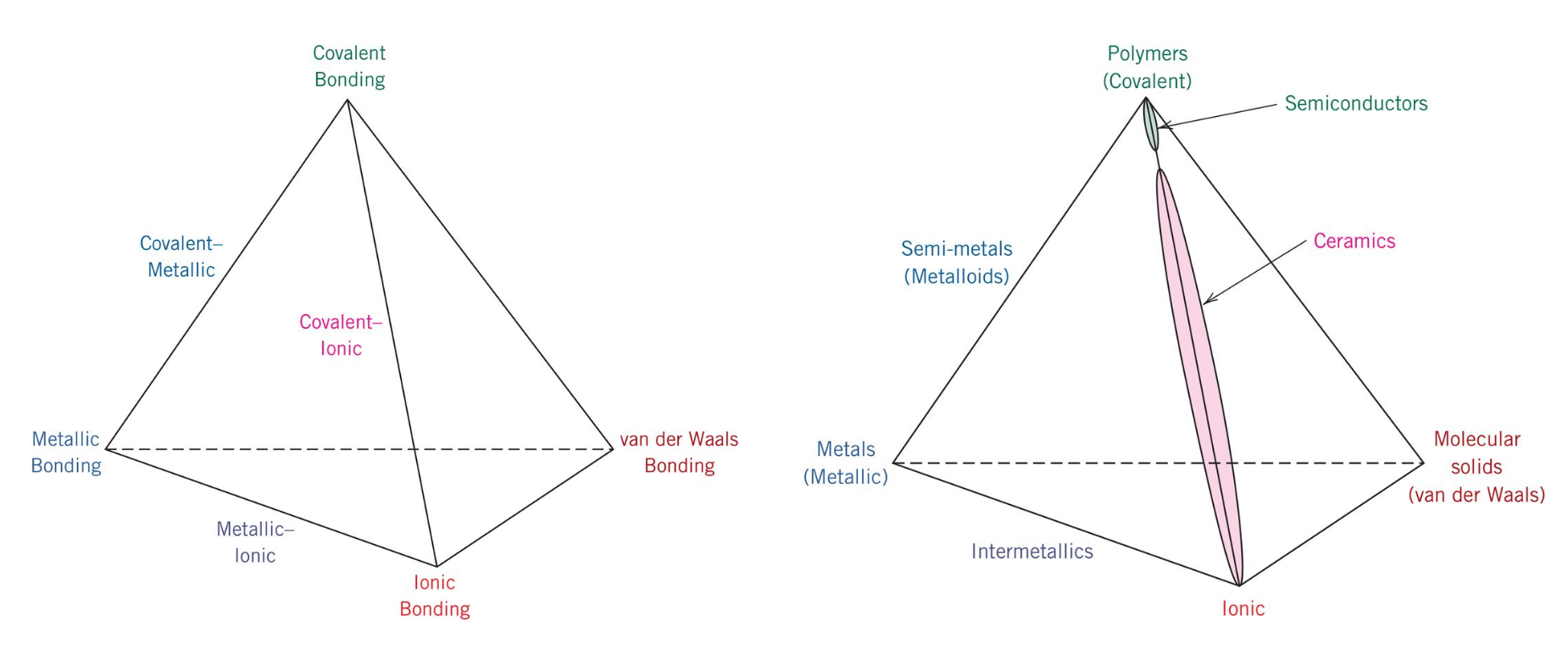
In addition, the reading noted a correlation between materials classification and bonding time. Ionic bonding is associated with ceramics, covalent bonding is associated with polymers, metallic bonding is associated with metals, and van der Waals bonding is associated with molecular solids. As we study materials in further detail in this course we will utilize these associations to explain observed materials properties in the different materials classifications. Before we proceed to this lesson’s video assignment, there are a couple of more topics that I would like to address. Your textbook highlighted water as a material of importance and its volume expansion upon freezing. We will explore this topic further in the next section.