Crude oil is a complex mixture of several types of hydrocarbon molecules along with inorganic impurities. These hydrocarbon molecules are:
- Paraffinic (or alkane series) hydrocarbons
- Naphthenic (or saturated cyclic) hydrocarbons
- Aromatic (or cyclic) hydrocarbons
- Asphaltene hydrocarbons
Sometimes we refer to a particular crude oil as a paraffinic crude oil or as an aromatic crude oil. While there may be several thousand different hydrocarbon molecules in a given crude oil, these descriptions simply refer to the dominant hydrocarbon type in the mixture. All naturally occurring crude oils will typically contain molecules of each type.
Paraffinic, or alkane series, hydrocarbons are shown in Table 2.01. These hydrocarbons are typically studied in introductory courses in Organic Chemistry. Alkane series hydrocarbons are composed of only hydrogen and carbon atoms attached with single bonds. They are characterized by the following formula for the number of hydrocarbon atoms present in the molecule:
In this formula, NH is the number of hydrogen atoms, while NC is the number of carbon atoms in the hydrocarbon molecule.
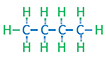
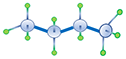
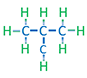
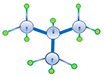
Normal alkanes are the chain molecules depicted the first four examples in Table 2.01. Once the number of carbon atoms reaches four (butanes) different permutations of an alkane molecule can exist that still honor Equation 2.01 but do not form chain molecules. These permutations are called isomers with the number of possible permutations (and number of isomers) increasing as the number of carbon atoms, NC, increases. An example of the difference between a normal and isomeric alkane is shown in Table 2.01 for n-butane and i-butane. Due to the slight discrepancies in the molecular structures, normal alkanes and isomeric alkanes have slightly different physical and chemical properties, such as, boiling points, melting points, etc.
| Compound | Formula | 2D Representation | 3D Representation |
|---|---|---|---|
| Methane | CH4 |
 |
 |
| Ethane | C2H6 |
 |
 |
| Propane | C3H8 |
 |
 |
| N-Butane Normal Butane |
C4H10 |
 |
 |
| I-Butane Isomeric Butane |
C4H10 |
 |
 |
As the number of carbon atoms increases, the molecular weight of the molecule increases. The lower molecular weight alkanes, methane and ethane, are the most common hydrocarbon components in natural gas; however, intermediate molecular weight alkanes up to the butanes may also be present in natural gases. In addition to these hydrocarbon components, some inorganic impurities, such as CO2, H2S, N2, and O2 may also be present in natural gases.
Crude oils from Pennsylvania are generally classified as Paraffinic Crude Oils (“Pennsylvania Grade Crude Oil” was at one time a phrase used to describe high quality crude oils.). As a solid, the alkanes form a waxy substance, paraffin, which is the main component of such products as paraffin candles.
The second category of hydrocarbon molecules found in crude oils are naphthenic (or saturated cyclic) hydrocarbons. Like the alkanes, these hydrocarbons are composed of hydrogen and carbon atoms attached with single bonds; however, they differ from the alkanes in that they do not form chain structures but form cyclic (ring) structures. Examples of naphthenic hydrocarbons are shown in Table 2.02.
| Compound | Formula | 2D Representation |
|---|---|---|
| Cyclopropane | C3H6 |
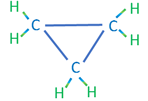 |
| Cyclobutane | C4H8 |
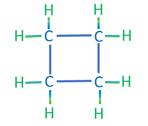 |
| Cyclopentane | C5H10 |
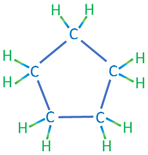 |
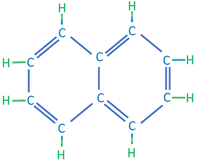
The third category of hydrocarbon molecules found in crude oils are the aromatic (or cyclic) hydrocarbons. These hydrocarbons are composed of hydrogen and carbon atoms that form cyclic structures but contain dual bonds between alternating carbon atoms. The simplest aromatic hydrocarbon is benzene with a single ring structure; while more complex aromatic hydrocarbons are typically formed with multi-ring structures. As the name implies, aromatic hydrocarbons, such as benzene, are associated with a sweet smell. Examples of aromatic hydrocarbons are shown in Table 2.03.
The last category of hydrocarbon molecules found in crude oils are the asphaltene hydrocarbons. These hydrocarbons are large, high molecular weight molecules which may also contain some atoms other than hydrogen and carbon atoms, such as, sulfur, oxygen, or nitrogen atoms. Asphaltenes typically result in the residue from the refining process and are the principal components of asphalt (road paving), tar, and bitumen products.
The mixture of these hydrocarbon molecules can have several consequences on oil and gas production, transport, and refining. These include:
- The mixture of hydrocarbon molecules gives the bulk fluids (oil and gas phases) the properties which dictate the ease or difficulty in which they flow through the reservoir towards production wells and through production equipment
- The nature of any solids which may precipitate from the bulk fluids onto rock grains, wellbore, or surface equipment during production causing operational problems requiring different solutions:
- Paraffinic crude oils may deposit waxy solids onto the reservoir rock, well completion, or production tubing causing restrictions to flow requiring a remediation treatment, such as hot kerosene injection into the well.
- Asphaltic crude oils may deposit hydrocarbon resins onto the reservoir rock, well completion, or production tubing causing restrictions to flow requiring a remediation treatment, such as solvent injection into the well.
- Local field treatment of the crude oil to upgrade it to the required specifications for the pipelines and refineries where the crude oil will eventually flow
- The products that can be refined from the crude oils once they reach the refineries: lubricating oils, heating oils, car or aviation fuels, asphalt and tar products, etc.
| Compound | Formula | 2D Representation |
|---|---|---|
| Benzene | C6H6 |
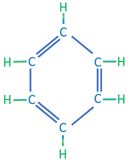 |
| Napthalene | C10H8 |
 |
The definitive methods for determining the different components in crude oil are with laboratory measurements, such as gas chromatography. Often, however, less rigorous methods may be useful for quick, on-site evaluations or for numerical (computer) calculations where the crude oil type is characterized with a single parameter. In the oil and gas industry, one common measure of the dominant character of the crude oil (paraffinic, naphthenic, and aromatic) is the Watson Characterization Factor[1] (or a generalization suggested by Whitson[2]). The original form of the Watson Characterization Factor, KW, is:
Where:
KW is Watson Characterization Factor, oR1/3
Tb is the mean average boiling point of the mixture, oR
ϒo is the specific gravity of the mixture, dimensionless
This equation requires that the boiling point, Tb, for the mixture of interest is known. In many cases, Tb is unknown or difficult to measure. For these cases, Whitson correlated the Watson Characterization Factor with the more commonly known molecular weight:
Where:
KW is Watson Characterization Factor, oR1/3
Mo is the molecular weight of the mixture, lbm/lbm-mol
ϒo is the specific gravity of the mixture, dimensionless
The guidelines for the use of the Watson Characterization Factor are:
- KW ≥ 12.5 indicates a paraffinic crude oil
- 12.5 > KW ≥ 10 indicates a crude oil containing naphthenic or aromatic components
- KW < 10 indicates a highly aromatic crude oil
Table 2.04 shows the values of the Watson Characterization Factor for substances of known hydrocarbon type (paraffinic, naphthenic, and aromatic).
| Hydrocarbon Series |
Substance | Formula | Tb (oR) |
Mo (lbm/lbm-mol) |
Υo |
Kw(oR1/3) Eq. 2.02a |
Kw(oR1/3) Eq. 2.02b |
|---|---|---|---|---|---|---|---|
| Paraffin | n-Hexane | C6H4 | 615.4 | 86.178 | 0.6640 | 12.8 | 12.7 |
| 2-Methylpentane (A) | C6H14 | 600.1 | 86.178 | 0.6759 | 12.8 | 12.8 | |
| n-Heptane | C7H16 | 668.8 | 100.205 | 0.6882 | 12.7 | 12.6 | |
| Napthene | Cyclohexane | C6H12 | 637.0 | 84.162 | 0.7834 | 11.0 | 11.0 |
| Methylcyclohexane | C7H14 | 673.4 | 98.189 | 0.7740 | 11.3 | 11.4 | |
| Aromatic | Benzene | C6H6 | 635.8 | 78.114 | 0.8844 | 9.7 | 9.8 |
| Toluene | C7H8 | 690.8 | 92.141 | 0.8718 | 10.1 | 10.2 |
(A) One of the isomers is Hexane