
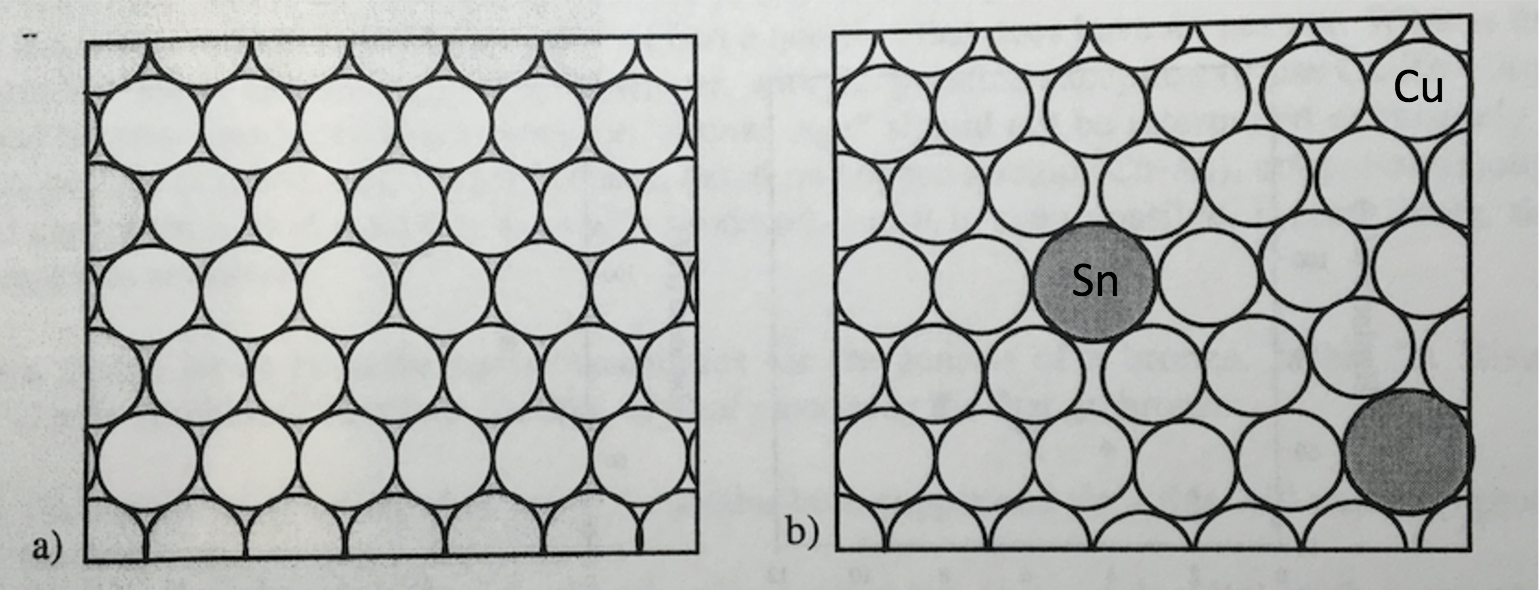
Bronze is an alloy of copper and tin. Tin is a slightly bigger atom than copper. In bronze, typically 5 – 10% is tin and the rest is copper. The slightly larger tin atoms replace copper atoms in the copper crystalline structure as shown in the figure below. We will learn more about metal alloys in this lesson and the next. Although copper and tin are both soft metals and not ideal for tools or weapons, the combination that produces bronze is much harder than copper or tin. As we will learn later, this is due to the larger tin atoms making it harder for rows of copper atoms to move. This results in bronze being harder.
In the practice of producing bronze, the Egyptians placed tin with copper ingots into clay crucibles. The clay crucibles were lowered into a charcoal fire which could exceed 1100 °C through the use of blowing air using foot bellows. The Egyptians would then stir, remove the slag, and pour the melt into a mold.