5.9 An Unusual Way to Make Precipitation in Mixed-Phase Clouds
Recall that water can exist in liquid form even below the freezing point. This supercooled liquid needs ice nuclei (IN) in order to become ice, although at a temperature of about –40 oC, the liquid can freeze homogeneously (without IN).
Recall from Lesson 4 that the vapor pressure over supercooled liquid water is greater than the vapor pressure over ice at the same temperature. So, if an ice particle is introduced into air that contains liquid water below the freezing point, the ambient vapor pressure in equilibrium with the liquid will be greater than the saturation vapor pressure of the ice. The ice will grow, but this uptake of water vapor will cause the ambient water vapor pressure to be less than the saturation vapor pressure for the liquid drops and the liquid drops will have net evaporation. This process will continue so that the ice grows at the expense of the liquid drops, which will shrink. The transfer of water is not by the liquid drops colliding with the ice crystal; the transfer of water comes from the liquid drops evaporating water to make water vapor and then that water vapor diffusing over to the ice, where it condenses. This process is called the Bergeron–Findeisen Process, and is a way that precipitation-sized drops can be formed in about 40 minutes in mixed-phase clouds (see figure below).
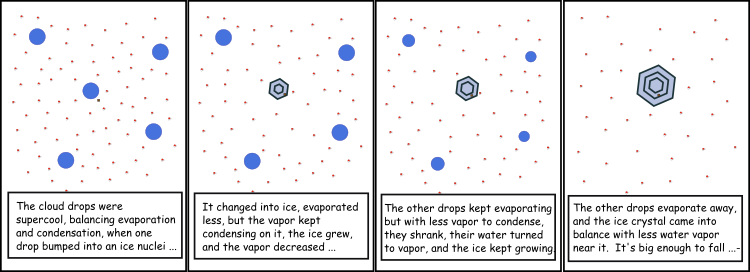
Frame 1: The cloud drops were supercool, balancing evaporation and condensation, when one drop bumped into an ice nuclei...
Frame 2: It changed into ice, evaporated less, but the vapor kept condensing on it, the ice grew, and the vapor decreased...
Frame 3: The other drops kept evaporating but with less vapor to condense, they shrank, their water turned to vapor, and the ice kept growing
Frame 4: The other drops evaporate away, and the ice crystal came into balance with less water vapor near it. It's big enough to fall...
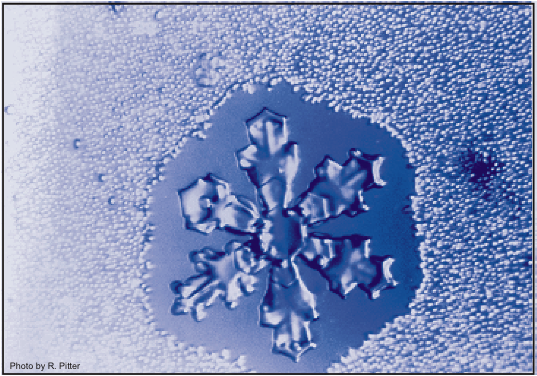
This process, as unusual as it seems, actually works, as can be seen in the figure below!