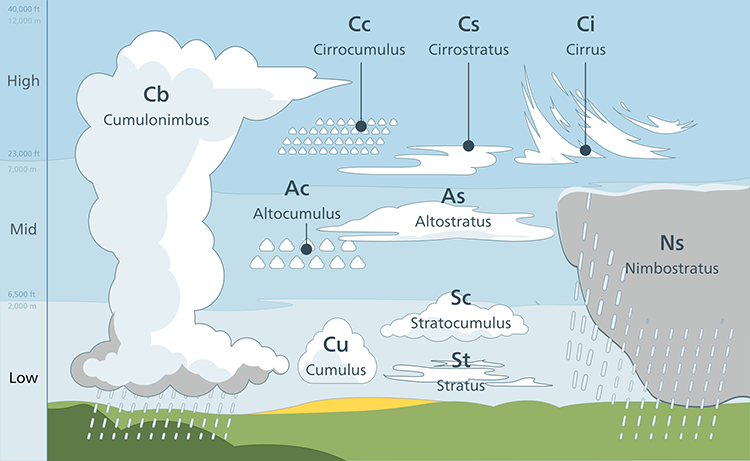
5.1 Do you recognize these clouds, drops, and snowflakes?
Clouds have fascinated people for millennia, but it wasn’t until 1802 that Luke Howard first classified clouds with the terms that are used today. His classification scheme was formalized later in the 19th century and has 10 basic cloud types with many minor variations (see figure below).
Clouds!
NOAA and NASA put together this thorough Sky Watcher Chart that describes a wide variety of cloud formations.
Cloud physics goes beyond the classification of clouds to determine the actual physical and chemical mechanisms that create clouds and cause their evolution over time. There are two aspects of cloud physics. One is the physics on the cloud scale, which is tens to hundreds of meters in size. This physics is driven in part by behavior in the cloud’s environment, such as the wind shear or the location of a front, and determines the evolution of the cloud and the cloud’s size and shape. All of this action, however, is not possible without the physics that is occurring on the microscale, which is less than a few centimeters in size.
This lesson deals mostly with the physics that occurs on the microscale and is often called cloud microphysics. Now that you are familiar with the concepts of thermodynamics and water vapor, we are ready to look at the fundamentals of cloud microphysics. To understand cloud-scale physics will require an understanding of atmospheric dynamics and turbulence, which are introduced in later lessons of this course.
A cloud is defined as a (visible) suspension of small particles in the atmosphere. For a water cloud, there are a number of types of particles that we are interested in.
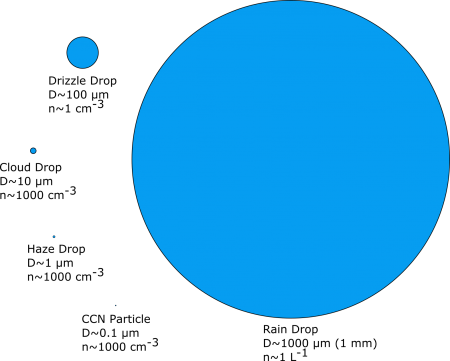
Rain Drop: D ~ 1000 μm, n ~ 1 L–1
CCN Particle: D ~ 0.1μm, n ~ 1000 cm–3
Haze Drop: D ~ 1μm, n ~ 1000 cm–3
Cloud Drop: D ~ 10 μm, n ~ 1000 cm–3
Drizzle Drop: D ~ 100 μm, n ~ 1 cm–3
Note the wide range in size, volume, and number of particles in the figure above. The smallest, the cloud condensation nuclei (CCN), can have rather little water and are made up of substances to which water can attach (called hydrophilic, water loving). The other particles grow by adding water molecules but still contain the original CCN upon which they formed.
We can specify the amount of water that is in liquid form by using the liquid water content (LWC), which is defined as:
Typical values of LWC are 0.1–0.9 g m–3, but a few g m–3 are possible for wetter conditions.
Check Your Understanding
A cloud drop is typically 5 µm in radius, while a raindrop, which comes from a collection of cloud drops, is typically 0.5 mm (500 µm) in radius. How many cloud drops does it take to make a raindrop?
ANSWER: Find the volume of the cloud drop and the volume of the raindrop and then find out how many times bigger the raindrop is. The answer is the number of cloud drops it takes to make a raindrop.
So we see that it takes about a million cloud drops to make one raindrop. Thus 109 cloud drops per m3 of cloud should make about 103 raindrops per m3 of cloud. This is about the number per m3 that are observed.
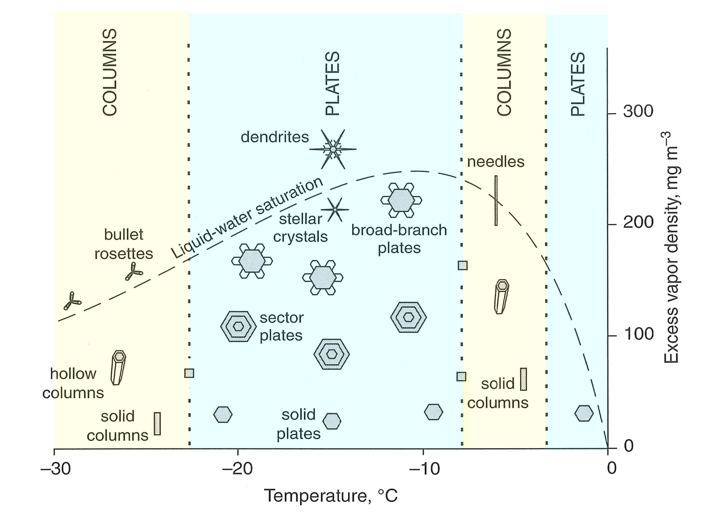
Ice crystal shapes for different temperatures:
Columns (–30 to –23 ºC):
Above the liquid-water saturation line: bullet rosettes
Below the liquid-water saturation line: hollow columns, solid columns
Plates (–23 to –8 ºC):
Above the liquid-water saturation line: dendrites
Below the liquid-water saturation line: stellar crystals, broad branch plates, sector plates, solid plates
Columns (–8 to –4 ºC):
On the liquid-water saturation line: needles
Below the liquid-water saturation line: solid columns
Plates (–4 to 0 ºC):
Below the liquid-water saturation line: Solid plates
Ice crystal habits as a function of temperature and excess water vapor (i.e., water vapor greater than saturation water vapor).

The next time it snows, catch snowflakes on a cold surface and take a good look at them. Their shape will tell you a lot about the environment in which they were formed. In State College, Pennsylvania, we often see plates with broad branches and sometimes we see dendrites, telling us that the snowflakes were formed at altitudes in the cloud where the temperature was between –22 oC and –8 oC and the excess vapor density was large.
The following video (3:52) entitled "Snowflake Safari" gives a simple explanation of snowflake formation and shows some nice pictures of different snowflake shapes.
FLORA LICHTMAN: Sure there's sledding, snowmen, skiing, but a winter storm can also mean safari. KEN LIBBRECHT: You really just need a snowy day. Take a magnifying glass, go out, there's all sorts of different things you can see. FLORA LICHTMAN: That's Ken Libbrecht, the physicist at Caltech who also happens to be a snowflake expert. He's been hunting flakes for years and documenting them before they melt with this microscope camera rig. KEN LIBBRECHT: My travel with that the hard part is getting it through airport security. FLORA LICHTMAN: Snow crystals come in roughly 35 flavors Libbrecht says. Some more common than others of course. KEN LIBBRECHT: Stellar dendrites are pretty common standard sort of shopping mall snowflake with six branches. FLORA LICHTMAN: Then there's the variant fern-like stellar dendrites. KEN LIBBRECHT: ...and they look like a little bitty ferns. FLORA LICHTMAN: Also common are... KEN LIBBRECHT: ..needles, columns. One of my favorites are capped columns. FLORA LICHTMAN: Which look kind of like a satellite or... KEN LIBBRECHT:...two wheels on axle. Unfortunately the most common thing you'll find is just kind of junky looking snow looks like sand. FLORA LICHTMAN: The least common, the ivory-billed woodpecker of snowflakes is big... KEN LIBBRECHT: ...five millimeters in diameter and nicely symmetrical with lots of intricate markings. Those are really gorgeous and hard to find. FLORA LICHTMAN: But you can increase your chances if you seek out snowflake hotspots. KEN LIBBRECHT: Northern Ontario is a good spot. Vermont and Michigan and I have been there. Northern Japan actually is pretty good. I'm anxious to try to Siberia. FLORA LICHTMAN: See certain conditions breed better crystals. KEN LIBBRECHT: The best temperature is around five degrees Fahrenheit. Sometimes though you can see it's really nice crystals just below freezing. FLORA LICHTMAN: Ok a little review of where snowflakes come from. They're born in the clouds. It all starts with a speck of dust or bacterium. KEN LIBBRECHT: Gunk in the air. FLORA LICHTMAN:...and the gunk floats around the cloud. KEN LIBBRECHT:...for half a mile. FLORA LICHTMAN: Picking up water molecules. KEN LIBBRECHT: Then they shuffle around a little bit until they find the right spot to sit in and that the water molecules themselves are lined up in the hexagonal array. That's where the the order is generated. FLORA LICHTMAN: ...and that order is what makes it a crystal. KEN LIBBRECHT:...and as a grows larger the points of the hexagon stick out a little bit in the air so each of the six corners sprouts and arm and that's one of the things we're trying to understand in details how crystals grow. FLORA LICHTMAN: The details of that growth are determined by the microenvironment, the flake encounters, as it travels through the cloud. KEN LIBBRECHT: Humidity is low the crystals grow slow and humidity is high they go fast. FLORA LICHTMAN: In other words of flakes identity is shaped by the environment it grows up in and because two snow crystals aren't likely to follow the exact same path, you're not likely to find two of the exact same flake. Just how environment affects crystal growth is something Librecht studies in the lab, by growing his own snowflakes. KEN LIBBRECHT: We call these designer snowflakes. You can sort of dial-up what you want. FLORA LICHTMAN: Give it the right environment and something to grow on and it'll build itself. KEN LIBBRECHT: A really nice example of how really complicated structures emerged spontaneously not alive test the DNA or anything like that genetic code. It just happens. To understand more about how works will be able to use it for something or at the very least we'll just understand how it works. FLORA LICHTMAN: Happy new year. For Science Friday, I'm Flora Lichtman.
Discussion Forum 3: Cloud identification
It's time to look up at the sky to observe the clouds. During the next week, take pictures of clouds and identify the clouds in the pictures. Try to focus on just one cloud type per image. Submit an image that depicts at least one cloud type.
This discussion will be worth 3 discussion points. I will use the following rubric to grade your participation:
| Evaluation | Explanation | Available Points |
|---|---|---|
| Not Completed | Student did not complete the assignment by the due date. | 0 |
| Student completed the activity with adequate thoroughness. | Student answers the discussion question in a thoughtful manner, including some integration of course material. | 1 |
| Student completed the activity with additional attention to defending their position. | Student thoroughly answers the discussion question and backs up reasoning with references to course content as well as outside sources. | 2 |
| Student completed a well-defended presentation of their position, and provided thoughtful analysis of at least one other student’s post. | In addition to a well-crafted and defended post, the student has also engaged in thoughtful analysis/commentary on at least one other student’s post as well. | 3 |