Proxy Techniques: Stable Isotopes, Trace Elements and Biomarkers
Oxygen Isotopes
Since we cannot travel back in time to measure temperatures and other environmental conditions, we must rely on proxies for these conditions locked up in ancient geological materials.
The most widely applied proxy in studying past climate change are the isotopes of the element oxygen. Isotopes refer to different elemental atomic configurations that have a variable number of neutrons (neutrally charged particles) but the same number of protons (positive charges) and electrons (negative charges). As you might remember from your chemistry classes, protons and neutrons have equivalent masses, whereas electrons are weightless. So, because different isotopes of the same element have different weights, they behave differently in nature.
Oxygen has three different isotopes: oxygen 16, oxygen 17 and oxygen 18. These isotopes are all stable (meaning they do not decay radioactively). O-16 is by far the most common isotope in nature, accounting for more than 99.8% of all oxygen atoms, and O-17 is exceedingly rare, but O-18 is abundant enough in nature to be measured. The masses of O-16 and O-18 are different enough that these isotopes are effectively separated by natural processes. This separation process is known as fractionation. Without going into too much detail, O-16 and O-18 are fractionated by the process of evaporation as well as when minerals, including shells of animals and plants, are precipitated from water. The main driver of the evaporation effect in most geological intervals is the amount of water that has been removed from the ocean and is sequestered in ice (see video clips below). Evaporation selectively removes the lighter isotope, O-16 from water leaving higher concentrations of the heavier isotope, O-18. Thus, shells and other materials formed in the ocean tend to have more O-18 during colder, glacial intervals than during warmer intervals. However, as a portion of the evaporate ends up falling as snow which is then converted to ice, the reverse holds for ice sheets, such as those in Greenland and Antarctica. Ice formed in glacial intervals has more O-16 than ice grown in warmer times. Superimposed on the evaporation effect is a temperature effect. Shells that grow in warmer water hold more O-16 than shells that grow in colder water, as explained in the following clips:
Video: Stable Isotopes 1 (1:34)
Oxygen has three major isotopes: oxygen 16, oxygen 17, and oxygen 18. All of these isotopes have eight protons, but oxygen 16 also has eight neutrons. Oxygen 17 has nine neutrons, and oxygen 18 has 10 neutrons. Because these substances have different weights, they behave differently in nature, and this allows environmental processes to fractionate them. By fractionation, I mean separation of the various I States by natural processes. And this fractionation allows geologists to use the isotopes as proxies. Here we consider the temperature proxy of oxygen isotopes. Oxygen 16 is much more abundant in nature than oxygen 18, but its mass provides for mass-dependent fractionation from oxygen 18. And this is a result of vibrational frequency differences that operate at different temperatures. For example, in the formation of calcite (CaCo3), warmer water will lead to the inclusion of more oxygen 16 in the calcite molecule. In colder water, more oxygen 18 will be included in the calcite molecule. This allows us to use the relative ratio of oxygen 16 and oxygen 18 in the calcite of foraminifera as a proxy for paleo temperature.
Video: Stable Isotopes 2 (1:42)
The second way in which oxygen isotopes are fractionated is via kinetic processes, for example, evaporation. Evaporation acts on oxygen isotopes because the light isotope of oxygen, oxygen-16, is more readily evaporated from water than oxygen-18. Thus, clouds contain more oxygen-16 than oxygen-18. And the rainfall that comes from these clouds also contains more oxygen-16 than oxygen-18, as does the snow that forms from the rain. When you have a warm climate without snow, most of this oxygen-16 that is included in these clouds evaporates, precipitates, and is returned to the oceans. When you have a cold climate, most of the oxygen-16 becomes locked up in ice. And therefore, the water, the seawater from which this evaporation ultimately drives, becomes more enriched in oxygen-18. Thus, when we have calcite--foraminiferal calcite-- that forms from seawater, in a cold climate, the foraminifera will tend to be enriched in oxygen-18. Whereas, in a warm climate, the foraminifera will tend to be enriched in oxygen-16. Likewise, when we have snow and ice forming in a cold climate, it will tend to be enriched in oxygen-16. Whereas, when we have a little bit of snow or ice forming in a warm climate, it will tend to be more enriched in oxygen-18.
A range of materials from the geological record demonstrates significant variations in their levels of O-16 and O-18 as a result of changes in climate. These changes are a combination of temperature and evaporation effects. The materials include shells of clams, corals, and plankton called foraminifera that inhabit the surface of the ocean, as well as cores taken from glacial ice that has formed at high latitudes (a term that describes the cooler regions closer to the poles) and high elevations, and even stalactites formed in caves. Later in this module, we will study the isotope records of several intervals of rapid climate change in the geological record.
Other temperature proxies: Mg/Ca and TEX-86
As we have seen, there are a number of assumptions that need to be made to convert oxygen isotope values to temperature. At times when there were major ice sheets, the proxy can be difficult to apply and in fact, most applications of oxygen isotopes during cold intervals focus on changes in ice volume, not warming or cooling. Thus, it is fortunate that there are other proxies that can be applied to determine temperature. Here, we consider two of these, the ratio of magnesium to calcium in fossil CaCO3 shells and the TEX-86 ratio in organic carbon.
Mg/Ca
Organisms that construct shells of the two CaCO3 polymorphs, aragonite and calcite, include magnesium in very small amounts in their shells. As it turns out that amount is heavily dependent on temperature as a result of somewhat complex kinetic processes. The higher the temperature, the more Mg is included in the shell. Unfortunately, there are a few complications that need to be considered. Like oxygen isotopes, the Mg/Ca proxy requires that all of the calcite or aragonite is original and that it has not been altered during burial of the shell. Second, the ratios appear to differ from one species to another, thus there have to be careful calibrations for individual species. These calibrations focus on developing a relationship between the Mg/Ca of the shell and the temperature of the water in which it grew. This can only be done in living obviously. For extinct species, assumptions need to be made and they result in some uncertainty. Workers are careful to restrict their Mg/Ca analysis to individual species. Third, the Mg/Ca ratio of seawater has changed slowly over time and this ratio can impact the absolute temperatures when the focus of study extends over many millions of years. The proxy is most useful when the study interval is short, the fossil shells are unaltered and the species are still living. Since Mg/Ca is not impacted by the volume of ice as are oxygen isotopes, the proxy is often used along with the latter system to determine the volume of glaciers in cold geologic period.
TEX-86
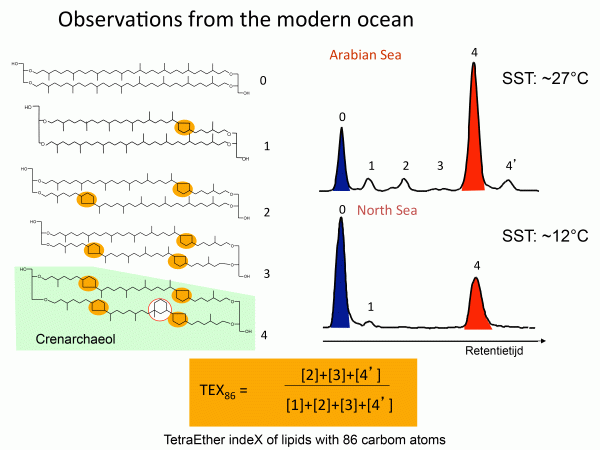
TEX-86 is a complex organic (meaning material that is composed largely of carbon, nitrogen, and phosphorus) proxy. This proxy is applied to compounds made by archaea or single-celled prokaryotes (organisms that do not have a differentiated nucleus). The archaea in question live in the oceans and are called crenarchaeota, and the TEX-86 proxy is based on changes in the composition of the lipid membranes of these organisms. The detailed structure of the membrane changes with temperature. The proxy is called TEX-86 because it is based on the tetraether index in carbon-86 atoms (you do not need to remember this!). TEX-86 has been calibrated in the modern oceans, meaning the indices of samples have been compared to the temperatures of the water in which they grow. The main uncertainty of the system revolves around the fact that the organisms of interest do not live right on the ocean surface so the temperatures theoretically are lower than surface levels. In addition, it appears that the index is impacted by the growth rate of the organism. Application of the TEX-86 is restricted to marine sediments and is most useful where there are no shells to measure for oxygen isotopes or Mg/Ca. Regardless of the uncertainties, the TEX-86 proxy is extremely useful to check results from these other two systems. Where two different proxies agree for the temperature of an interval the results are much more certain.
Carbon Isotopes
Oxygen is not the only abundant element with isotopes that can be used as environmental proxies. The isotopes of the element carbon also are used as proxies in environmental reconstruction. Carbon has a number of isotopes including C-14, which is radioactive, and two stable (i.e. non-radioactive) isotopes, C12 and C13. C-14 are widely applied in dating recently formed natural materials that contain significant amounts of carbon such as shells, charcoal, and even materials that contain trace amounts of carbon such as pots and cloth, since its abundance can be accurately determined using an accelerator, and its rate of decay is rapid.
Video: Stable Isotopes 3 (1:40)
The three main isotopes of carbon are carbon-12, carbon-13, and carbon-14. Carbon-12 has six protons and six neutrons, carbon-13 has six protons and seven neutrons, and carbon-14 has six protons and eight neutrons. Carbon-14 is a radioactive isotope and disappears after a hundred thousand years. In addition, carbon-12 is much more abundant in nature than carbon 13. Because Carbon-12 and carbon-14 have different atomic weights, these isotopes are fractionated via a number of different biological processes. The main process that fractionates carbon-12 in nature is photosynthesis. Because carbon-12 is much lighter than carbon-13, the organic material formed via photosynthesis is enriched in carbon 12, and the material from which this organic material forms, the atmosphere or the ocean, remains enriched in the other isotope, carbon-13. Other processes that fractionate carbon-12 and carbon-13 include respiration and the formation of methane. Because biological processes operate over time and fractionate carbon-12 and carbon-13, the ratios of carbon-12 and carbon-13 in natural materials is changed significantly over time and this fractionation has a number of different applications, as we will see later in the module.
However, because of its rapid decay, C-14 disappears in a short time (about 100,000 years). The isotopes C-12 and C-13 are not radioactive, are common in natural materials, and are fractionated by environmental processes in a way that they can be applied as proxies. C-12 has 6 protons and 6 neutrons and is the most abundant of all of the isotopes of carbon. C-13 has 6 protons and 7 neutrons and is much less abundant than C-12, but still can be measured by mass spectrometry (the technique used to measure the abundance of stable isotopes). C-12 and C13 are strongly fractionated during photosynthesis, when plants convert CO2 and sunlight into food; it requires less energy for a plant to incorporate an atom of the lighter isotope C-12 than it does an atom of C-13. In fact, when plant material is formed via photosynthesis, thousands of times more C-12 is incorporated than is C-13. As we will see, carbon isotopes are key indicators of the sources of greenhouse gas in the atmosphere, both today and in the past. C isotopes can be used as proxies for nutrient cycling and deep water circulation, as well as the source of plant materials.
