Ocean - Atmospheric Exchange
Carbon dioxide can be dissolved in seawater, just as it can be dissolved in a can of soda. It can also be released from seawater, just as the CO2 from soda can also be released. This transfer of gas back and forth between a liquid and the atmosphere is an extremely important process in the global carbon cycle, since the oceans are such an enormous reservoir with the potential to store and release significant quantities of CO2.
The exchange of a gas like CO2 between the air and seawater is governed by the differences in concentrations, as shown in the figure below, where the solid red line represents the concentration (increasing to the right) in the air and in the ocean. The dashed line indicates what the concentration might look like after some exchange of CO2 occurs — lower concentration in the atmosphere, and higher in the oceans. But note that the change in concentration is less in the ocean than in the atmosphere, which is a result of some chemistry we will explore in just a bit.
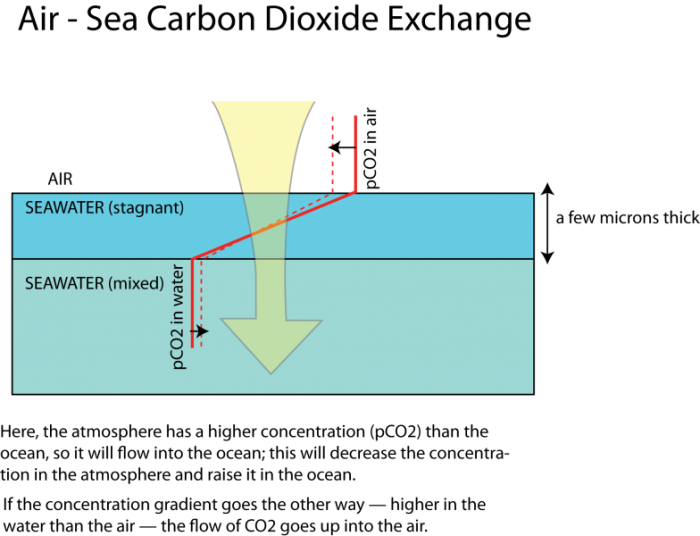
This image illustrates the movement of gases between two layers of seawater. The image is divided into two horizontal sections, both depicting seawater but with different properties, against a solid black background.
- The top section has the text "SEAWATER (stagnant)" in black on the left and is a light blue layer. At the top of this section, a yellow triangular shape points downward, representing a source of gases.
- The bottom section has the text "SEAWATER (mixed)" in black on the left and is a slightly darker cyan layer.
- A red dashed line runs diagonally from the top right of the stagnant layer to the bottom left of the mixed layer, indicating the boundary between the two layers.
- A black arrow with the text "O2" points downward from the yellow triangle, passing through the stagnant layer, across the red dashed line, and into the mixed layer, showing oxygen moving from the stagnant to the mixed seawater.
- Another black arrow with the text "pCO2" points downward alongside the O2 arrow, following the same path, indicating the partial pressure of carbon dioxide also moving from the stagnant to the mixed layer.
The image uses contrasting colors and clear labels to highlight the gas exchange between the two seawater layers. A text description at the bottom says: Here, the atmosphere has a higher concentration (pCO2) than the ocean, so it will flow into the ocean; this will decrease the concentration in the atmosphere and raise it in the ocean. If the concentration gradient goes the other way–higher in the water than the air–the flow of CO2 goes up into the air.
As depicted in the figure above, the concentrations in the air and the sea are relatively constant (spatially, not temporally) since these two media undergo rapid and turbulent mixing that would tend to even out any systematic variations. The exception to this is a thin layer of water, just 20 to 40 microns thick (a micron is one thousandth of a millimeter), which, because of the surface tension of the water, is unable to mix well. This stagnant film is the barrier across which the diffusion has to occur. The rate of gas transfer is a function of the concentration difference and the thickness of the stagnant film of water (which thins when the winds are strong).
In general, this flow depends on the concentration of CO2 in the atmosphere and the oceans, but it gets a little complicated because the concentration of CO2 in seawater depends on a number of other factors. To understand this process, and to see why the atmosphere's concentration changes more than the ocean, we need to have some sense of what happens to CO2 once it gets dissolved in seawater.
Carbonate Chemistry of Seawater
When CO2 from the atmosphere comes into contact with seawater, it can become dissolved into the water where it undergoes chemical reactions to form a series of products, as described in the following:
- CO2 (dissolved gas) + H2O <=> H2CO3 (carbonic acid)
- H2CO3 (carbonic acid) <=> H+ + HCO3- (hydrogen ion + bicarbonate)
- HCO3- <=> H+ + CO3-2 (hydrogen ion + carbonate)
The amount of CO2 as dissolved gas is what controls the concentration of CO2 shown in the figure above. This concentration depends strongly on the temperature — it is low in cold water and it is high in warm water, which means that the colder parts of the ocean absorb CO2 from the atmosphere and the warm parts of the ocean release CO2 into the atmosphere.
All of these reactions mean that in seawater, you can find all of these different forms or species of inorganic carbon co-existing, dissolved in seawater. In reality, though, bicarbonate (HCO3-) is the dominant form of inorganic carbon; carbonate (CO32-) and dissolved CO2 are important, but secondary (see figure below).
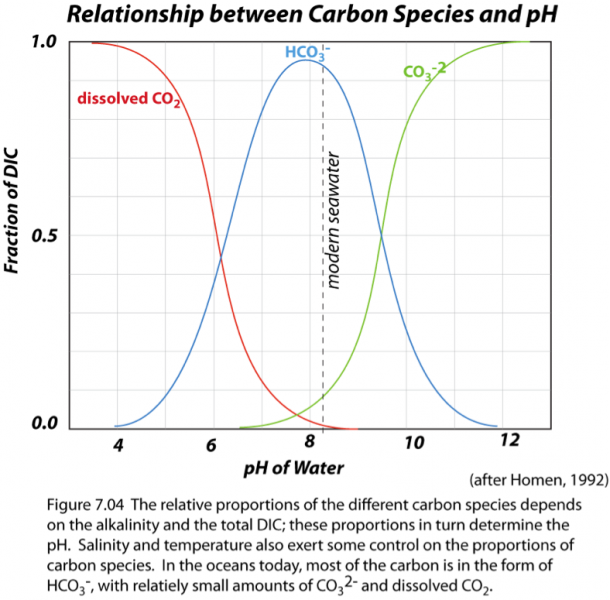
The image is a graph titled "Relationship between Carbon Species and pH." It shows the fraction of Dissolved Inorganic Carbon (DIC) on the y-axis (ranging from 0.0 to 1.0) versus the pH of water on the x-axis (ranging from 4 to 12). Three curves represent different carbon species:
- A red curve labeled "dissolved CO2" peaks around pH 5 and decreases sharply as pH increases.
- A green curve labeled "HCO3-" rises from pH 4, peaks around pH 8, and then declines.
- A blue curve labeled "CO32-" starts near zero at pH 4, increases steadily, and dominates at pH values above 10.
A vertical dashed line at pH 8.04, labeled "modern seawater," intersects the curves. Below the graph, text reads: "pH = 7.04. The relative proportions of the different carbon species depends on the alkalinity and the total DIC; these proportions in turn determine the pH. Salinity and temperature also exert some control on the proportions of carbon species. In the oceans today, most of the carbon is in the form of HCO3⁻, with relatively small amounts of CO32- and dissolved CO2. (after Homen, 1992)". A text description in the image says: The relative proportions of the different carbon species depends on the alkalinity and the total DIC; these proportions in turn determine the pH. Salinity and temperature also exert some control on the proportions of carbon species. In the oceans today, most of the carbon is in the form of HCO3-, with relatively small amounts of CO3 (2-) and dissolved CO2.
In the above equations (1-3), the double-headed arrows mean that the reactions can go in both directions, and generally do, until some balance of the different compounds is achieved -- a chemical equilibrium.
Notice that in the equations above, the hydrogen ion appears in a number of places — the concentration of H+ in seawater is what determines the pH of the water, or in other words, its acidity. Remember that low pH means more acidic conditions. It turns out that the ratio of bicarbonate (HCO3) to carbonate (CO3) is proportional to the pH; at lower pH conditions, more of the carbon is in the form of bicarbonate than carbonate, and the result is that the fraction of dissolved CO2 gas also goes up, and this tends to cause CO2 gas to move from the seawater into the atmosphere. As we will see in Module 7, higher acidity (lower pH) also has important implications for organisms that live in the sea.
Obviously, the ratio of these two forms of carbon is important, so we need to ask what controls this. Without getting into the chemistry too much, the ratio depends on two main things, and it depends on these things because they determine the electrical charge balance in the water — the charges from all the positive and negative ions dissolved in the water have to add up to zero, and you can see that if you have more carbonate (minus 2 charge) than bicarbonate (minus 1 charge), you have more total negative charge. So, the 2 important factors here are:
- the total amount of positive charge in the oceans that has to be canceled by the carbonate and bicarbonate — called the alkalinity;
- the total amount of dissolved inorganic carbon in the oceans (DIC for short).
If the alkalinity is high, then more of the DIC has to take the form of carbonate (minus 2 charge), and a result is that the pH is higher, meaning less acidic. If the total DIC is large, then to get the charges to balance, more of the DIC has to be in the form of bicarbonate (minus 1 charge), which leads to lower pH and more acidic conditions.
Let’s return now to the figure that showed the exchange of CO2 between the atmosphere and ocean and the question about why the ocean’s CO2 concentration changed less than the atmosphere. The reason for this is the carbon chemistry reactions that shift some of the CO2 dissolved gas into the forms of bicarbonate and carbonate — this reduces the amount of carbon in the form of dissolved CO2 gas, so the concentration of dissolved CO2 does not increase as much as it would if these reactions did not take place.
As you can see, the chemistry of carbon in seawater is relatively complex, but it turns out to be extremely important in governing the way the global carbon cycle operates and explains why the oceans can swallow up so much atmospheric CO2 without having their own CO2 concentrations rise very much.
Summary of Carbonate Chemistry
Let's see if we can summarize this carbonate chemistry — it is important to have a good grasp of this if we are to understand how the global carbon cycle works.
- Carbon can exist in three main inorganic forms in seawater — CO2, HCO3-, and CO32-, and there is a rapidly-achieved equilibrium between these species.
- The ratio of HCO3- to CO32- along with the water temperature determines the CO2 concentration of seawater and also the pH.
- The temperature of the water also controls how much CO2 occurs in the form of dissolved gas, thus affecting the concentration of CO2 gas in seawater.
- The alkalinity of seawater represents the positively-charged ions that need to be countered by negatively-charged carbonate and bicarbonate ions.
- The concentration of the total dissolved inorganic carbon (DIC), along with the alkalinity, determines the ratio of HCO3- to CO32-, and thus the CO2 concentration of seawater. If we increase DIC without changing the alkalinity, then more carbon must be in the form of HCO3-, which increases both pH and the CO2 concentration of seawater.
- The CO2 concentration of seawater, relative to the atmospheric CO2, determines whether the oceans absorb or release CO2. Currently, the cold parts of the oceans absorb atmospheric CO2 and the warm regions of the oceans add CO2 to the atmosphere.
- The ability of carbon to switch back and forth between these three forms means that only a portion of the CO2 absorbed by the oceans will remain as CO2.
In the real world, there are important variations in the gas transfer between the ocean and atmosphere. This can be seen in the figure below, which represents a kind of snapshot of this transfer across the globe. The units here are grams of C per m2 per year, and each box is about 1e6 m2. The red, orange, and yellow colors represent places where the oceans are giving up CO2 to the atmosphere; the blue and purple areas are places where the oceans are sucking up atmospheric CO2. Summing these up, we find that the oceans are taking up around 92-93 Gt C/yr and they are releasing about 90 Gt C/yr — for a net flow of 2-3 Gt C/yr into the oceans — this represents something like 25-30% of the carbon we are adding to the atmosphere by burning fossil fuels. This exchange is variable in space and time, but a few general features can be pointed out. In general, the colder parts of the oceans absorb CO2 and the warmer parts release CO2 into the atmosphere. This makes sense because CO2 is more soluble in colder water.
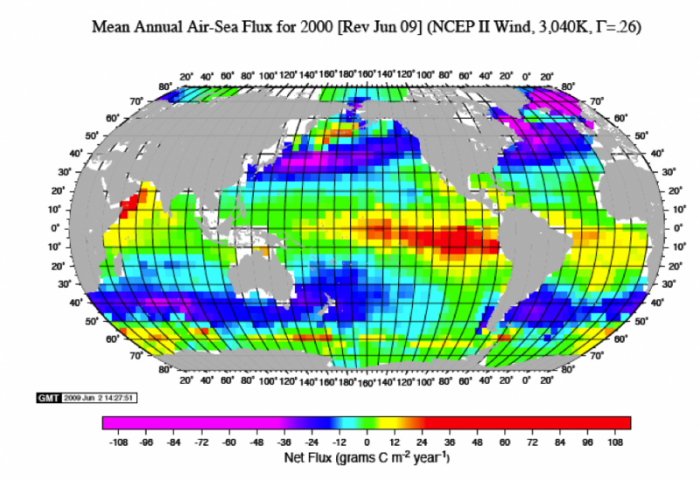
The image is a world map titled "Mean Annual Air-Sea Flux for 2000 [REV Jun 09] (NCEP II wind, 3,040K, T=-26)." It shows the net flux of carbon (in grams C m2 yr-1) between the atmosphere and the ocean for the year 2000. The map uses a color gradient to represent flux values, with a scale at the bottom ranging from -108 (deep blue) to +108 (deep red), passing through green (around 0).
- The map is overlaid with a grid of latitude and longitude lines, with latitude marked from 80°N to 80°S and longitude from 180°W to 180°E.
- Oceans are colored based on carbon flux:
- Areas in deep blue (e.g., parts of the North Atlantic, Southern Ocean) indicate strong carbon uptake by the ocean (negative flux, up to -108 g C m2 yr-1).
- Areas in red (e.g., equatorial Pacific, parts of the Indian Ocean) indicate carbon release to the atmosphere (positive flux, up to +108 g C m2 yr-1).
- Green areas (e.g., parts of the mid-latitudes) indicate near-neutral flux (close to 0 g C m2 yr-1).
- Landmasses, such as North and South America, Africa, and Australia, are shown in gray.
- A timestamp in the bottom left corner reads "2000-Jan 2 14:37:51."
The map visually highlights regions where the ocean acts as a carbon sink or source, with significant uptake in high-latitude regions and outgassing in equatorial zones.
