Prioritize...
After completing this section, you should be able to describe transmission, absorption, and scattering as they pertain to electromagnetic radiation passing through a medium. You should also be able to define albedo and be able to discuss earth's average albedo and that of various surfaces.
Read...
Unlike the traveler in Robert Frost's poem, The Road Not Taken, electromagnetic radiation doesn't have much of a choice whenever it encounters objects in its direct path. Indeed, the fate of electromagnetic radiation depends on wavelength and the physical composition of the atoms and molecules in the medium that it is passing through. It is impractical (and impossible) to sort through each atom and molecule in a given object in order to judge its potential effect on the radiation that strikes it ("incident" radiation), so we will consider chunks of matter as whole objects in order to describe their overall effect on incident radiation.
When radiation first encounters some medium (whether it be a collection of gases, a liquid, or a solid), only three things can occur to that radiation. The electromagnetic energy can either be absorbed by the medium, scattered by the medium, or it can pass through the medium unaffected (a process called transmission). In most cases, all three processes can and do occur to some degree. Examine the figure below showing the three processes that can affect radiation passing through a medium.
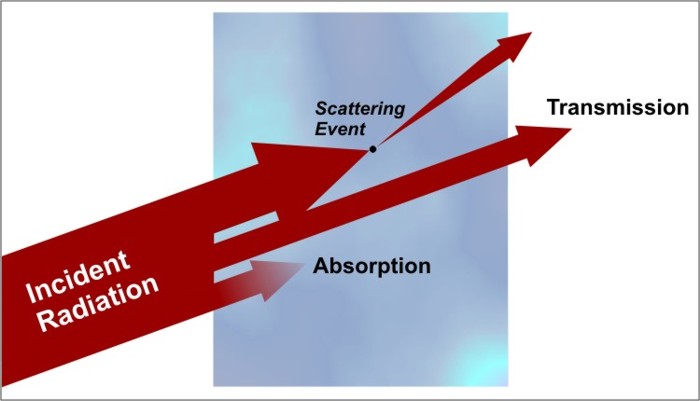
Let's briefly discuss each of these potential outcomes:
- Transmission is essentially the process by which radiation passes through an object unaffected. An example of a medium with a high transmission value is window glass. Visible light passing through a thin sheet of glass does so basically undisturbed, which is why we can see objects clearly on the other side. We tend to call such mediums "transparent," while mediums having low transmission values are called "opaque." The transmission properties of a medium are highly dependent on wavelength, however. For example, an object that is transparent in the visible wavelengths might be opaque in some infrared wavelengths. I should also point out that 100 percent transmission is rare, except within the vacuum of space. Almost always, at least a little energy is lost to absorption and/or scattering as radiation moves through the medium.
- Absorption is the extinguishing of a portion of the radiation "beam." When an object absorbs electromagnetic radiation, the radiation is taken up by the matter (typically the electrons of the atoms) and converted to other forms of energy (usually heat energy). As with transmission, the amount of energy that an object absorbs depends on the wavelength of the radiation and the physical make-up of the object. For example, freshly fallen snow absorbs little direct sunlight, but snow readily absorbs infrared radiation.
- Scattering occurs when radiation interacts with matter in a way that changes its direction of "travel." Scattering can occur in all directions, although some directions are preferred, depending on the size and composition of the particles involved in the scattering event. If the radiation encounters a scattering event and continues on in a forward direction, the event is called "forward-scattering." Likewise, objects can also back-scatter radiation, meaning that they redirect the radiation in all directions back toward the source. In some rare cases, the scattered radiation may retain the exact same direction that it initially had before the scattering event. When this occurs, the scattered light is counted in the "transmission" category (because it seemingly emerged unchanged from the medium).
Now let's see these processes (particularly absorption and scattering) in action in the atmosphere. First, the atmosphere, like snow, is a highly discriminating absorber (it only absorbs certain wavelengths of the electromagnetic spectrum). The plot of absorption spectra by various gases (below) indicates how efficiently certain gases and the atmosphere, taken as a whole, absorb various wavelengths of electromagnetic radiation. To interpret the graph, note the "0 to 1" scale on the left of the plot, indicating zero percent absorption and 100 percent absorption, respectively. At any specific wavelength, the upward reach of the color shading indicates the percentage of absorption by a particular gas (or the atmosphere, taken as a whole).
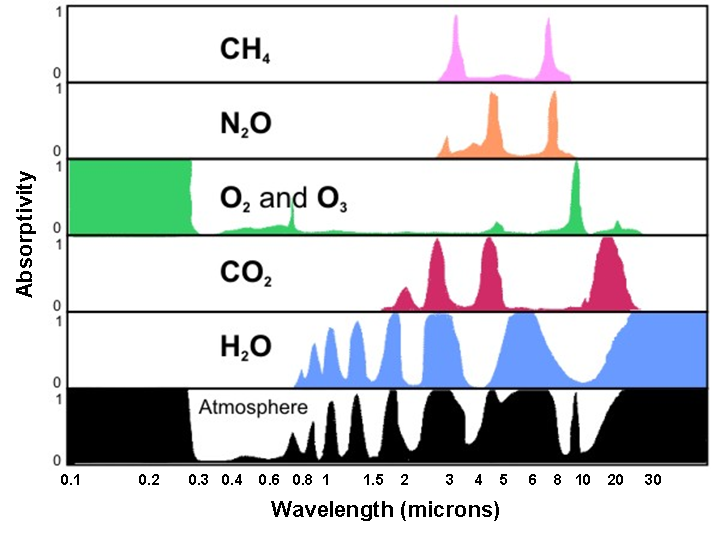
For example, focus your attention on the row for oxygen and ozone, labeled "O2 and O3." Note, to the left of this label, that nearly 100 percent of the radiation emitted at wavelengths ranging from 0.1 to about 0.3 microns is absorbed. Recall that these wavelengths correspond to potentially dangerous ultraviolet radiation emitted by the sun. Ozone, a gas composed of three oxygen atoms (O3), absorbs much of the incoming ultraviolet radiation in the stratosphere, which is a layer that spans from 10 to 30 miles above the Earth's surface. Thank goodness for ozone in the stratosphere! Otherwise, cases of skin cancer and other afflictions associated with overexposure to the sun would likely be much more rampant in our society than they actually are.
Scattering, on the other hand, makes things look the way they do. You can't see objects if visible light isn't scattered to your eyes. But, scattering doesn't have to be a one-time event. Often, radiation will enter an object and encounter many (hundreds/thousands) of scattering events before emerging. This is what happens to make clouds appear white on top and darker on the bottom (cue the obligatory storm photo). It's also what makes snow, salt, sugar, and milk white. Furthermore, multiple scattering increases the time that the radiation resides in the medium (as it bounces around unable to escape). This longer residence time increases the chance that the radiation will also be absorbed by the medium. A great example is the blue hue that ice sometimes develops. Water (even in frozen form) tends to absorb wavelengths associated with red light at a faster rate than those associated with blue light, so over time with multiple scattering events, more blue light is scattered to our eyes (see below)!

Keep in mind that all three processes (transmission, absorption, and scattering) often occur to some degree when radiation passes through a medium. For example, let's focus on what happens when solar radiation encounters earth's atmosphere:
- about 30 percent is reflected back to space by air molecules, clouds, and the earth's surface. Note that I'm using the word "reflection" as a loose substitute for "back-scattering," but there's a big difference between this loose use of "reflection" and the classic, pure interpretation of "reflection." Pure reflection means that the angle at which radiation strikes an object must equal the angle at which the radiation is redirected from the object (think about how a billiard ball bounces off a bumper on a pool table).
- about 20 percent gets absorbed by clouds and atmospheric gases
- roughly the remaining half is transmitted and ultimately absorbed by the earth's surface
So, of the solar radiation reaching Earth's atmosphere, about half never even makes it to the surface. It turns out we have a special name for the portion that is reflected--albedo, which simply refers to the fraction of radiation striking a surface that is reflected (again, using "reflected" loosely). So, Earth's albedo, on average, is about 30 percent, but albedo over parts of the earth varies from location to location and time to time. For example, thick thunderstorm clouds can have an albedo greater than 70 percent. Snow cover also has a high albedo because it reflects much of the solar radiation that strikes it (fresh snow cover can have an albedo greater than 90 percent). On the other hand, surfaces like asphalt parking lots, forests, or ocean water tend to have low albedos (less than 30 percent) because they don't reflect much of the solar radiation that strikes them.
Obviously, for us here on Earth, the sun is a major source of radiation, but it's not the only important source. Indeed, clouds and atmospheric gases are sources of radiation, too, and they play a critical role in the flow of energy through the earth-atmosphere system. Read on.