Prioritize...
Once you've completed this page, you should be able to discuss the so-called "greenhouse effect," and the "greenhouse gases" that contribute to it, as well as its importance for life on Earth. You should also be able to describe the connection between the greenhouse effect and global warming and make a distinction between the two.
Read...
I hope that over the last few sections you've gotten the idea (which maybe surprised you initially) that the atmosphere itself is an important contributor to Earth's energy budget. That's right, even invisible atmospheric gases (and clouds) emit some radiation toward the earth's surface! The key to understanding this observation lies in our laws of radiation. Recall that Planck's Law tells us that all objects emit radiation at all wavelengths at all times.
As you've learned, the earth's peak emission occurs at infrared wavelengths (from Wien's Law), so what happens to that radiation after it's emitted upward from the surface? Some is absorbed by air molecules, in particular, so-called "greenhouse gases," such as water vapor, carbon dioxide, methane, and nitrous oxide. Of the greenhouse gases, water vapor is the most abundant in the atmosphere, followed by carbon dioxide (although recall that in the overall scheme of the atmosphere, these are trace gases). It turns out that some of the wavelengths that carbon dioxide and water vapor absorb readily (particularly those around 15 microns and a little larger) coincide with the wavelengths of earth's peak emission.
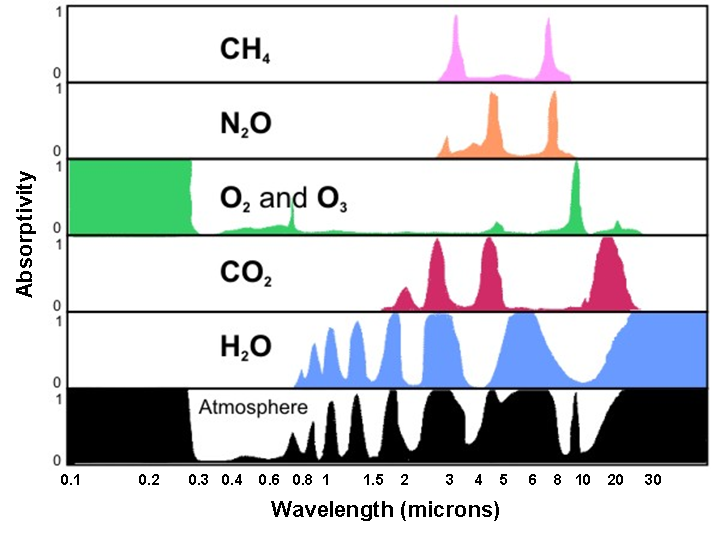
Kirchoff's Law tells us that if an object is an efficient absorber of radiation at a particular wavelength, then it's also an efficient emitter of radiation at that wavelength. A consequence of Kirchoff's Law then is that greenhouse gases like water vapor and carbon dioxide also emit IR radiation efficiently at those wavelengths, some of which is emitted toward Earth's surface. The emissions that reach the surface are a major contributor to the "downwelling infrared" traces on the graphs we were using for our energy budgets.
The fact that greenhouse gases absorb and emit infrared radiation so readily works out very well for humans. Without emissions of downwelling IR from greenhouse gases, the average temperature of Earth's surface would be about 0 degrees Fahrenheit (-18 degrees Celsius). That's a pretty harsh environment for life on earth, and certainly life as we know it could not exist. However, observations show that the average temperature of Earth's surface is about 59 degrees Fahrenheit (15 degrees Celsius), and it's downwelling IR from greenhouse gases that are responsible. Without greenhouse gases, the temperature of Earth's surface would be nearly 60 degrees lower -- much, much colder!
The contributions of downwelling IR from greenhouse gases to warming the planet are called the greenhouse effect. To be honest, the names "greenhouse effect" and "greenhouse gases" are pretty unfortunate, because the processes at work to create the planetary warming are not the same as those in a greenhouse, but I'll touch on that shortly. The bottom line is that the warming from the greenhouse effect is essential to sustaining life as we know it on Earth.
Global Warming
The existence of earth's greenhouse effect is perhaps as important as its distance from the sun in determining the average global surface temperature, so there's no doubt that some greenhouse effect is desirable. But, can we have too much of a good thing? Might the magnitude of the greenhouse effect change if we change the concentration of greenhouse gases in the atmosphere?
Before the Industrial Revolution in the late 1700s, the atmospheric concentration of carbon dioxide was around 280 parts per million, but through the burning of fossil fuels like coal, oil, and natural gas, humans have added carbon dioxide to the atmosphere. The concentration of carbon dioxide in the atmosphere now exceeds 400 parts per million, and you can see the upward trend in atmospheric carbon-dioxide concentration since the late 1950s in the data from the Mauna Loa Observatory in Hawaii below.
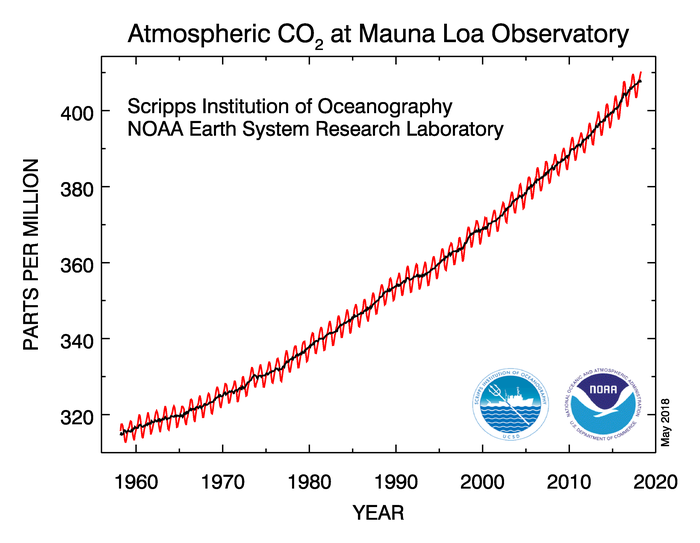
Remember that carbon dioxide is the second most important greenhouse gas (behind water vapor) so increasing its concentration gradually results in a stronger greenhouse effect, which means more downwelling IR being emitted toward Earth, causing the planet to warm additionally (a "global warming"). So, if you've read an article or watched a news story about global warming, the strengthening of the greenhouse effect from an increased concentration of greenhouse gases is the basic science behind it. That's far from the whole picture, though, and we'll explore other issues related to global warming and climate change in a later lesson.
Finally, I mentioned earlier that the phrases "greenhouse effect" and "greenhouse gases" are rather unfortunate because the processes involving emission of radiation from gases is a different process than what keeps a greenhouse warm. The name "greenhouse effect" was dubbed in the early 1800s when it was thought that greenhouses stayed warmer because the panes of glass allowed solar radiation to enter, but prevented radiation emitted from plants and other objects inside the greenhouse from escaping. It turns out that a big reason why greenhouses stay warmer inside has to do with the fact that the air inside cannot mix with cooler air outside the greenhouse. The warm air in a greenhouse essentially gets trapped inside the panes of glass, but there is no "trapping" with respect to the atmospheric "greenhouse effect" (even though you may still see it described in terms of "trapping heat"). The atmospheric "greenhouse effect" is all about the absorption and emission of infrared radiation by some atmospheric gases. But, alas, the name "greenhouse effect" stuck, and the rest is history.
The idea of air not being able to mix with cooler air outside a greenhouse leads us to our next topics. Indeed, we've talked a lot about how energy is transferred via radiation, but it's time to look at how energy is transferred through the earth-atmosphere system by contact between objects and by the movement of air (the very movement of air prevented by the panes of glass on a greenhouse). Read on.