Prioritize...
At the completion of this section, you should be able to define radiation, wavelength, and micron. You should also be able to discuss the organization of radiation in the electromagnetic spectrum by wavelength (you should know which types of radiation have longer wavelengths and which have shorter wavelengths, for example).
Read...
First up in our study of Earth's energy ledger is radiation. While the mention of "radiation" may conjure up thoughts about nuclear reactors or nuclear bombs, it turns out that the scientific use of the term "radiation" is considerably more broad. Radiation is defined as the emission and transfer of energy through a medium via particles or waves. In fact, the vast majority of radiation that you encounter on a daily basis has nothing to do with nuclear radiation at all. From an everyday light-bulb, to the microwave that heats your frozen lunch, to the radio that you listen to on your morning commute, you're surrounded by devices that make use of radiation. Even light from the sun is a form of radiation, so radiation is occurring all around you!
At some point in a science class, you probably studied the electromagnetic spectrum of radiation, but how is this electromagnetic spectrum created? To begin with, you probably know that the building blocks of all matter are atoms and molecules. Within these atoms and molecules are smaller particles which have positive and negative charges -- protons and electrons, respectively. These charged particles tend to oscillate or vibrate (especially electrons). Without getting into the details, physics tells us that any charged particle like an electron has an electrical field surrounding it (electrical charges and electrical fields go hand-in-hand). Furthermore, moving charges also possess magnetic fields. Thus, when an electron oscillates, its surrounding electric and magnetic fields change. Like moving your hand rapidly back and forth in a pool of water, oscillating electrons send out ripples of energy (that is, "waves") that have both electrical and magnetic properties (hence, electro-magnetic radiation).

So, how is it that different kinds of electromagnetic waves exist to create an entire spectrum? First, let's talk about the different kinds of EM radiation in terms of wavelength. The wavelength of any wave is simply the distance between two consecutive similar points on the wave (for example, from wave crest to wave crest). Now think about our pond analogy above. If you move your hand slowly in the water, you will create a few waves with long wavelengths. However, if you move your hand rapidly in the water, you create lots of waves with very short wavelengths. The same is true for an oscillating electron. If the oscillation is very quick (we say the oscillation has a high frequency), then the EM radiation produced will have a short wavelength. If the oscillation is slower (having a lower frequency) then the electromagnetic waves will have long wavelengths.
Now, the frequency at which electrons oscillate is essentially set by the temperature of the matter in which the electron resides (remember, we defined an object's temperature as the average kinetic energy of its atoms or molecules). The higher the temperature, the higher the frequency of oscillation. So, when temperature increases, the wavelength of the electromagnetic radiation emitted by the electron decreases. Conversely, as temperature decreases, the frequency of oscillation slows, and the wavelength of the emitted electromagnetic radiation increases. For a visual, check out this short video demonstrating the relationship between oscillation frequency and wavelength (0:57)
Let’s explore a simple model of how oscillation frequency is tied to the wavelength of electromagnetic radiation.
The frequency at which electrons oscillate is essentially set by the temperature of the matter in which the electron resides. Lower temperatures yield lower frequencies of oscillation. Here, we’ve set our temperature on the low side, and you can see the molecule oscillating fairly slowly, or in other words, at a low frequency. The wavelength of the emitted radiation is also relatively long.
But, when temperature increases, the oscillations get faster, which makes for a higher oscillation frequency. This high-frequency means that the emitted electromagnetic radiation has a relatively short wavelength. For comparison again, we can decrease our temperature to watch the oscillation frequency slow, and the wavelength of the emitted radiation increase.
Before we move on, let me add one quick caveat: the brief description I gave above doesn't fully describe all emissions of radiation. For simplicity, we focused on the generation of EM radiation by a single oscillating charged molecule. In reality, matter exists as a system of charged particles. This means that the resulting electromagnetic radiation field is much more complex than I have outlined here. Furthermore, very high-frequency EM emissions require a different mechanism to generate the high-energy waves in addition to moving particles, but that's beyond the scope of what we need for this course.
With that caveat out of the way, check out the entire spectrum of electromagnetic radiation below. First, note that the range in wavelengths for different types of electromagnetic radiation is staggering -- from hundreds of meters to the size of an atom's nucleus. Also note that visible light does indeed qualify as electromagnetic radiation, despite taking up only a tiny sliver of the entire spectrum. This means that our eyes are completely blind to almost all electromagnetic radiation.
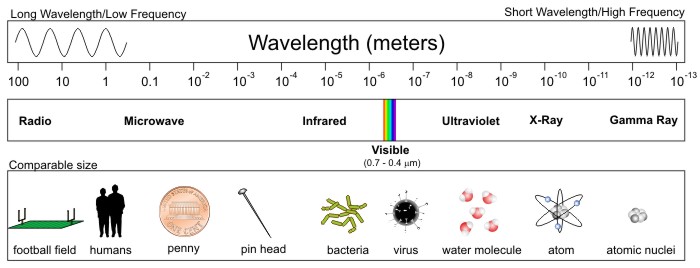
Starting from the end of the spectrum with the longest wavelengths (the "long-wave" portion of the spectrum), radio waves and microwaves have wavelengths of hundreds of meters to a few millimeters (a millimeter is 10-3 meters or one-thousandth of a meter). As wavelengths further decrease, they tend to be expressed in micrometers, more commonly called microns (10-6 meters, or one-millionth of a meter), and as wavelengths shrink to 10s of microns (the size of a bacterium or a virus) we label these emissions as infrared, visible, and ultraviolet light. Finally, in the very short-wave portion of the spectrum, with wavelengths the size of an individual molecule or atom, we have X-rays and gamma rays.
The types of radiation that we'll be working with most commonly in this course are microwave, infrared, and visible because of their applications in helping meteorologists observe the atmosphere. Furthermore, as we'll investigate more in this lesson, beyond the longest wavelengths associated with visible light lies the infrared ("beyond red") band of the electromagnetic spectrum. A majority of the infrared spectrum, spanning from approximately 3 to 100 microns, essentially constitutes "terrestrial radiation" (radiation "of the earth") because the oscillating charges that emit at these wavelengths are consistent with temperatures commonly observed on this planet as well as in Earth's atmosphere.
Now that you know the terminology behind the different regions of the electromagnetic spectrum, we need to discuss the properties by which objects emit radiation. These properties have been grouped into what I call the "four laws of radiation." Read on.