Prioritize...
When you've finished this page, you should be able to describe energy transfer via convection, as well as discuss ways to generate convection such as buoyancy and mechanical eddies.
Read...
Our final method of energy transfer is convection. First I'm going to define convection and describe two ways to generate it, and then we'll apply that knowledge to see why winds help inhibit the development of nocturnal inversions.
Convection
While conduction can be a painfully slow method of energy transfer, convection is more like a speedy locomotive. Convection is the transfer of heat energy via the vertical movement of the air. Remember those very thin layers of air in contact with paved surfaces on hot summer days? They can approach 140 degrees Fahrenheit thanks to conduction, but convection limits the thickening of those blazing hot layers of air. Just like a hot-air balloon lifting off the ground, blobs or "parcels" of hot air rise from the ground, carrying hot air skyward. This transfer of heat energy away from the ground by the vertical movement of air is called "free convection" or "natural convection."

To understand the nuts and bolts of free convection, let's start with the concept of buoyancy. Suppose that, while taking a swim, you submerge your favorite beach ball and then let it go. In a heartbeat, the beach ball will bob to the surface of the water. In scientific terms, the beach ball is positively buoyant. Now submerge a rock and then release it. It falls to the bottom of the pool because the rock lacks sufficient positive buoyancy to keep it afloat. Formally, we say that the rock has negative buoyancy.
What makes the difference in the buoyancy between a rock and a beach ball? The answer is density. Formally, the density of an object is its mass (akin to weight) divided by its volume. The beach ball has a relatively large volume and small mass, making its density rather small and far less than the density of water. A rock, on the other hand, has a greater density than water, so it sinks. We can generalize these observations in this way: an object immersed in a fluid (water, air, etc.) is positively buoyant if the density of the object is less than the density of the fluid. Moreover, the magnitude of the buoyancy force depends on the difference in densities between the submersed object and the fluid - the greater the difference, the greater the buoyancy force.
Okay, let's take our discussion out of the water and into the air. For the time being, let's start with a "parcel" of air ("parcel" is just a fancy name for a generic blob of air that we assume does not interact with surrounding air). Several factors can cause the density of the air to change, but we're going to focus on the effects of temperature on air density.
To understand the connections between changes in temperature and changes in density, let's conduct a simple experiment. First, I'll place a soda bottle in a pan of cold water for a few minutes and then cover the opening with a cheap party balloon. With the balloon sealing off the air in the bottle, we've isolated a "parcel" of air with a constant mass (provided we don't remove the balloon). Now, I'll heat the container of water in which the bottle sits. As the water warms the bottle and the inside air, air molecules increase their kinetic energy, and the air inside the bottle expands and inflates the balloon. In other words, the volume occupied by the "air parcel" is now larger, despite the mass of the air remaining the same. It follows that the air density, which is mass divided by volume, is now less.

Our experiment gives us an important result: Increasing the temperature of the air inside a parcel causes its density to decrease (and vice versa). In turn, the positive buoyancy of the parcel increases and, as a result, it shows a tendency to rise if it's "submersed" in air with higher density.
Now let's connect this discussion on density and buoyancy to free convection. On a sunny day, the sun heats the ground and, in turn, the ground heats a thin layer of air in contact with it by conduction. But the ground heats the overlying air unevenly, so there are spots that are hotter than others. For example, think about a sunny summer day and the torridly hot air in contact with the concrete surface of a parking lot. Now think about the cooler air that overlies the surrounding grassy area. The air over the parking lot is less dense than the surrounding air and therefore more positively buoyant. In turn, air parcels rise more readily from the parking lot, transferring heat energy upward. This transfer of heat energy is, of course, a consequence of free convection.
Manifestations of free convection vary from sensational cumulonimbus clouds (thunderstorm clouds) that reach miles into the sky to the "thermals" routinely ridden by hawks, glider pilots, and hang gliders. To the naked eye, these thermals, which are currents of rising air associated with free convection, are often invisible. However, a striking form of imaging called Schlieren photography (explanation) allows for us to visualize the rather subtle variations of density of air parcels rising from a relatively warm object like the ground. Below is a Schlieren photo showing convection from a former meteorology instructor at Penn State, Lee Grenci. Notice the thermals of warm buoyant air that can be seen rising from his skin. You can really see the thermals in this Schlieren video showing more of Lee's "heat". A Transcript for this Visualizing Convection video is here.

The turbulent swirls of air shown in the image above (also shown in the video) are called eddies, and whether we can see them or not, they're the essence of convection. But, free convection that results from positively buoyant air parcels isn't the only source of convective eddies. Indeed, when the wind blows over the rough surface of the earth, it creates turbulent eddies. These eddies result because friction slows wind speeds near the earth's surface, but higher up, wind speeds are faster because friction is weaker. Faster winds blowing over slower winds causes eddies to develop. You can simulate this idea yourself if you place a pencil in the palm of your hand and then slide your other hand over the pencil. The pencil rolls, doesn't it? That's the basic process that allows the wind to churn up convective eddies, which meteorologists call "mechanical convection" to differentiate it from free convection.
It is ultimately mechanical convection generated by wind that can prevent the formation of nocturnal inversions, even on clear nights. At night, invisible eddies mix colder air in contact with the cooling ground upward, while also circulating slightly warmer air toward the ground from higher up. To help you visualize the mixing effects of eddies, check out this (28 second, silent) video from the Department of Mechanical Engineering at the University of Melbourne. Researchers were looking at how eddies are formed in a fluid flowing over a surface, which is very similar to what happens when air blows over the ground. Notice how eddies mechanically mix the light and dark layers of the fluid. You can think of the light-colored fluid as cold air in contact with the ground, while the dark-colored fluid is warm air above the ground. With time, you can see the eddies mix the two fluids--the same process that occurs on a windy night.
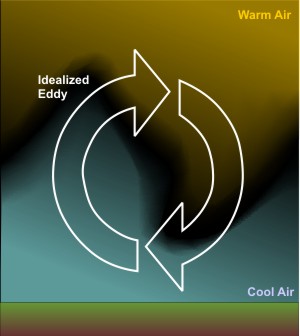
As the speed of the wind increases, eddies become more turbulent and more vigorously circulate air upward to an altitude of several thousand feet. Eddies try to run a balanced budget and, in compensation, circulate air toward the ground from similar altitudes, effectively satisfying the popular adage that "what goes up must come down." In addition to the speed of the wind, the local roughness of the earth's surface also determines the upward reach of eddies.
In windy conditions, the ups-and-downs associated with turbulent eddies thoroughly stir and mix the lower atmosphere. What is the impact on temperature? Well, when you add cold milk to a cup of hot coffee and mix the two, the resulting fluid has a temperature that is warmer than the cold milk but cooler than the hot coffee, and so it is with mixing from eddies. Mixing from eddies keeps air near the surface warmer than it would be if winds were calm and conduction was allowed to dominate. So, all other meteorological factors being equal, a windy night is warmer than a calm night. This result may seem contrary to your experience because a windy night often "feels" cooler than a calm night, but that chilly feeling of the wind results from an accelerated loss of body heat.
As I mentioned before, eddies from free convection (and/or mechanical convection, if it's windy) on sunny, summer days are also responsible for limiting the thickness of those blazing hot, thin layers of air in contact with the surface. As hot air parcels become positively buoyant and rise, other parcels sink and bring cooler air toward the surface. So, thank goodness for the mixing from eddies from both mechanical and free convection!
Now that we've covered all the modes of energy transport, we're going to wrap up the lesson by debunking a commonly heard myth about clouds and blankets. Keep reading!