The Greenhouse Effect and the Global Energy Budget
Earlier, we noticed that if you do the energy balance calculation to figure out the temperature of our planet, it suggests that Earth should be -19 °C, which is 34 °C colder than the observed average global temperature of 15 °C. Why is Earth warmer than it should be? The answer lies in the greenhouse effect — gases in our atmosphere (including CO2, CH4 (methane) and H2O water vapor) trap much of the emitted heat and then re-radiate it back to Earth’s surface. This means that the energy leaving our planet from the top of the atmosphere is less than one would expect given the known temperature of our planet. As mentioned earlier, this effect can be represented in the simple energy balance equation as a term called the emissivity.
The fact of this greenhouse effect comes out of the very simple calculation we did above, but it can also be observed in great detail from satellite measurements of the infrared energy leaving Earth’s atmosphere.
As we discussed on the topic of black body radiation, the temperature of a body (a planet, for instance) gives us a sense of what the spectrum of energy should look like — that is, a range of wavelengths and intensity of radiation at those wavelengths. For the Earth, this spectrum, as seen from satellites looking down on the surface, is very different from the expected. The figure below shows the difference between the expected and the observed.
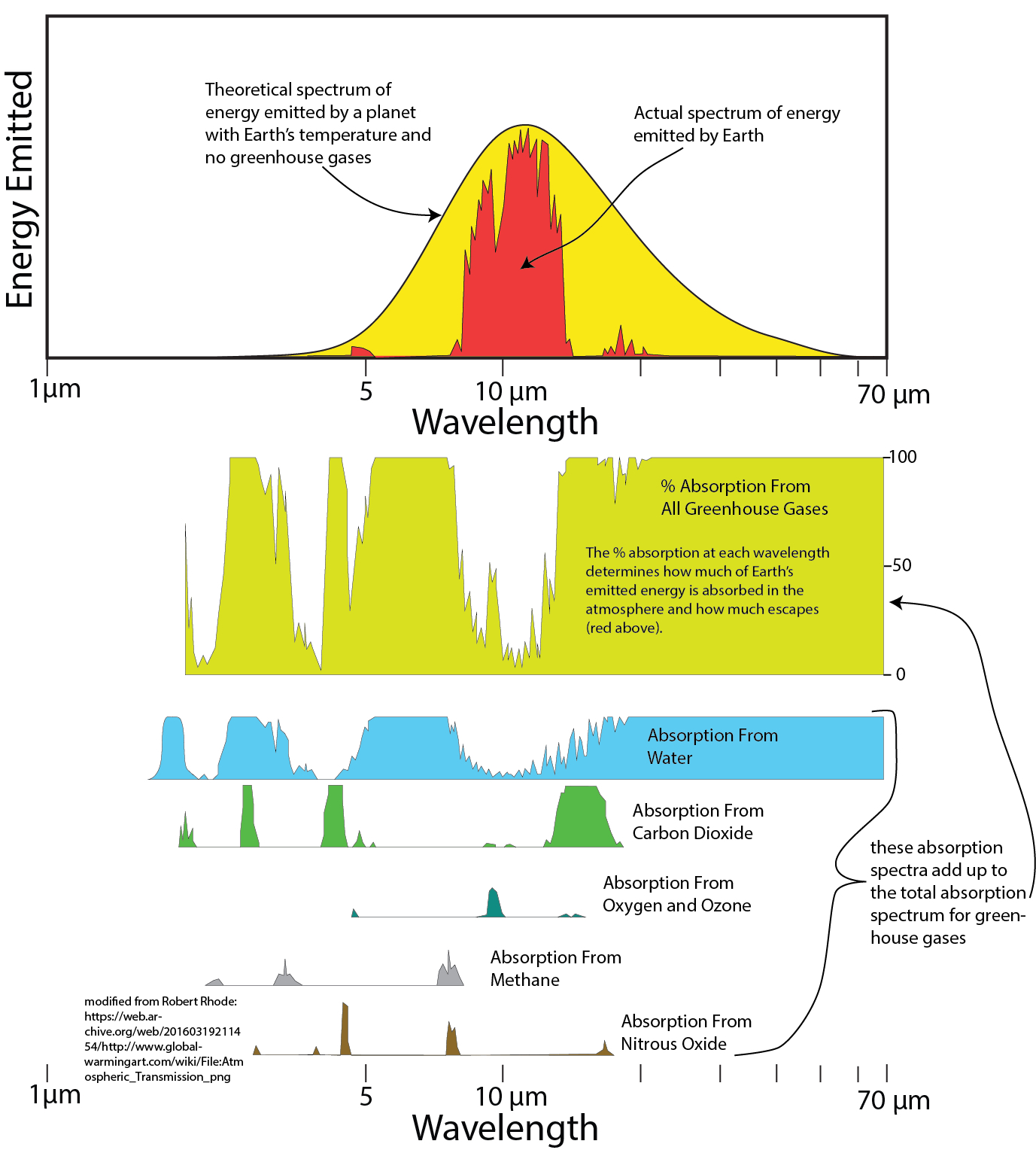
The image consists of two related diagrams illustrating the spectrum of energy emitted by Earth and the absorption of this energy by greenhouse gases across different wavelengths. The diagrams highlight the role of greenhouse gases in trapping Earth's emitted energy, contributing to the greenhouse effect. The image is credited to Robert Rhode and includes a source link.
- Top Diagram (Energy Emission Spectrum):
- Axes:
- X-Axis (Wavelength): Labeled "Wavelength," ranging from 1 μm (micrometer) to 70 μm, with major ticks at 1, 5, 10, and 70 μm.
- Y-Axis (Energy Emitted): Labeled "Energy Emitted," with no specific units provided, but the scale is relative, showing the intensity of emitted energy.
- Data Representation:
- Yellow Curve: Represents the theoretical blackbody emission spectrum for a planet at Earth's temperature (~288 K) without greenhouse gases, peaking around 10 μm.
- Red Curve: Represents the actual spectrum of energy emitted by Earth, showing significant reductions at certain wavelengths due to absorption by greenhouse gases.
- The area between the yellow and red curves is shaded red, indicating the energy absorbed by the atmosphere.
- Trend: The yellow curve follows a smooth blackbody radiation curve, while the red curve shows dips at specific wavelengths where greenhouse gases absorb energy, reducing the amount of energy escaping to space.
- Axes:
- Bottom Diagram (Absorption by Greenhouse Gases):
- Title and Label: The bottom diagram shows the "% Absorption From All Greenhouse Gases" and breaks down the absorption contributions from individual gases.
- Axes:
- X-Axis (Wavelength): Matches the top diagram, ranging from 1 μm to 70 μm, with major ticks at 1, 5, 10, and 70 μm.
- Y-Axis (Absorption): For the topmost graph, labeled "% Absorption From All Greenhouse Gases," ranging from 0 to 100%, with major ticks at 0, 50, and 100%.
- Data Representation:
- Topmost Graph (Yellow with Blue Shading): Shows the percentage of Earth’s emitted energy absorbed by all greenhouse gases combined (blue shading) at each wavelength, with the red curve from the top diagram overlaid to show the emitted energy that escapes.
- Individual Gas Absorption Graphs: Below the combined absorption graph, separate graphs show the absorption spectra for specific greenhouse gases:
- Water Vapor (Blue): Strong absorption around 5–7 μm and beyond 20 μm.
- Carbon Dioxide (Green): Significant absorption around 4 μm and 15 μm.
- Oxygen and Ozone (Gray): Absorption primarily around 9–10 μm.
- Methane (Brown): Absorption around 3.5 μm and 7–8 μm.
- Nitrous Oxide (Dark Brown): Minor absorption around 4.5 μm and 8 μm.
- A label on the right side reads: "these absorption spectra add up to the total absorption spectrum for green-house gases," indicating that the combined absorption (topmost graph) is the sum of the individual contributions.
- Trend: The absorption graphs show that different gases absorb energy at specific wavelengths, with water vapor and carbon dioxide being the most significant contributors to the overall absorption.
The diagrams together illustrate how greenhouse gases absorb Earth’s outgoing infrared radiation, reducing the energy that escapes to space and contributing to the greenhouse effect. The absorption spectra of individual gases highlight their specific roles in this process.
In fact, the same thing happens to the energy the Earth receives from the Sun — various gases in the atmosphere absorb that energy, so the amount we receive on the surface is less than what arrives at the top of the atmosphere.
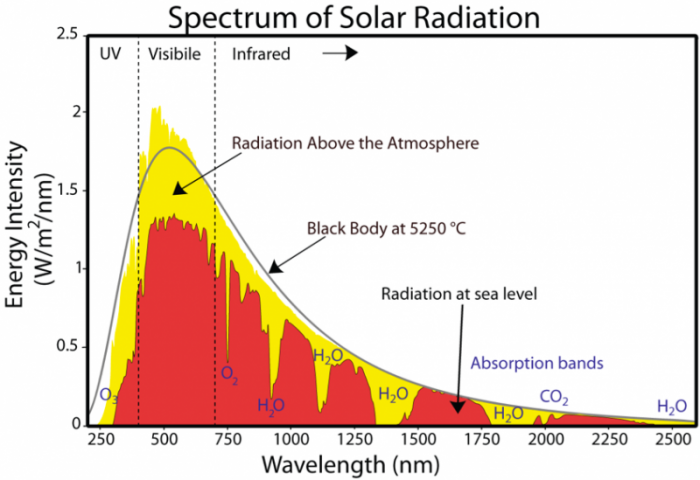
The image is a graph titled "Spectrum of Solar Radiation," showing the energy intensity of solar radiation across different wavelengths, comparing the radiation above the atmosphere to that at sea level. It highlights the effects of atmospheric absorption by various gases.
- Title: The title at the top reads: "Spectrum of Solar Radiation."
- Axes:
- X-Axis (Wavelength): Labeled "Wavelength (nm)," ranging from 250 to 2500 nanometers (nm), with major ticks at intervals of 250 nm (250, 500, 750, 1000, 1250, 1500, 1750, 2000, 2250, 2500).
- Y-Axis (Energy Intensity): Labeled "Energy Intensity (W/m2/nm)," ranging from 0 to 2.5 watts per square meter per nanometer (W/m2/nm), with major ticks at intervals of 0.5 (0, 0.5, 1.0, 1.5, 2.0, 2.5).
- Data Representation:
- Two curves are plotted:
- Radiation Above the Atmosphere: Represented by a smooth yellow curve labeled "Radiation Above the Atmosphere," also referred to as "Black Body at 5250°C." This curve approximates the Sun's emission as a blackbody at 5250°C (approximately 5523 K, close to the Sun's surface temperature of ~5773 K).
- Radiation at Sea Level: Represented by a red curve labeled "Radiation at sea level," showing the solar radiation after passing through the atmosphere.
- The area between the yellow and red curves is shaded red, indicating the energy absorbed by the atmosphere.
- Two curves are plotted:
- Spectral Regions:
- The graph is divided into three regions along the x-axis:
- UV (Ultraviolet): From 250 nm to ~400 nm.
- Visible: From ~400 nm to ~700 nm.
- Infrared: From ~700 nm to 2500 nm.
- These regions are marked with vertical dashed lines separating UV, visible, and infrared.
- The graph is divided into three regions along the x-axis:
- Absorption Bands:
- Specific absorption bands are labeled along the red curve, indicating where atmospheric gases absorb solar radiation:
- O₃ (Ozone): Absorbs strongly in the UV range (around 250–350 nm).
- O₂ (Oxygen): Absorbs around 750 nm.
- H₂O (Water Vapor): Absorbs at multiple wavelengths, notably around 900 nm, 1100 nm, 1400 nm, and 1900 nm.
- CO₂ (Carbon Dioxide): Absorbs around 2000 nm.
- These absorption bands cause dips in the red curve, showing reduced energy intensity at sea level compared to above the atmosphere.
- Specific absorption bands are labeled along the red curve, indicating where atmospheric gases absorb solar radiation:
- Curve Characteristics:
- Yellow Curve (Above Atmosphere): Peaks around 500 nm in the visible range at an intensity of about 2.0 W/m2/nm, then gradually decreases toward longer wavelengths, reaching near 0 W/m2/nm by 2500 nm.
- Red Curve (At Sea Level): Follows the yellow curve but with significant reductions at specific wavelengths due to absorption. It peaks slightly below 2.0 W/m2/nm around 500 nm and shows pronounced dips corresponding to the absorption bands of O3, O2, H2O, and CO2.
- Overall Trend:
- The graph illustrates that while solar radiation above the atmosphere follows a smooth blackbody curve, the radiation reaching sea level is significantly altered by atmospheric absorption.
- The visible range (400–700 nm) experiences relatively less absorption, allowing most of the sunlight in this range to reach the surface, while UV and infrared regions are more heavily absorbed by atmospheric gases.
The graph effectively demonstrates the impact of Earth's atmosphere on incoming solar radiation, highlighting the role of specific gases in absorbing energy at different wavelengths.
How do gases absorb this energy? It is basically a matter of vibrations of gas molecules being in sync with some of the frequencies of energy associated with insolation or infrared energy given off by Earth. You can think of the bonds between atoms in an H2O molecule like springs that stretch, twist, and bend at specific frequencies (nice animation of H2O movement), and if energy hits those molecules at just the right frequency, the bonds of the molecule absorb that energy and oscillate and stretch and twist more strongly.
There are numerous ways to demonstrate this heat-trapping ability of some gases — here is a nice laboratory demonstration of heat-trapping — but you can also think of the difference between the cold nighttime temperatures when the air is dry (little water vapor) compared to the warmer nighttime temperatures when the air is humid. The fact of the greenhouse effect is one of the most important things to understand about our climate system. This greenhouse effect, which is probably better described as warming produced by heat-trapping gases, is incredibly powerful — it returns more energy to the surface than we absorb from the Sun, and its strength is closely tied to the global carbon cycle, and thus the oceans, and all the biota on Earth.
Let’s try to put a lot of this together now and have a glance at the energy budget for Earth’s climate. The figure below attempts to illustrate where all the energy goes in the climate system. We start with 100 units of energy, which represents the total amount of energy Earth receives from the Sun in a year.
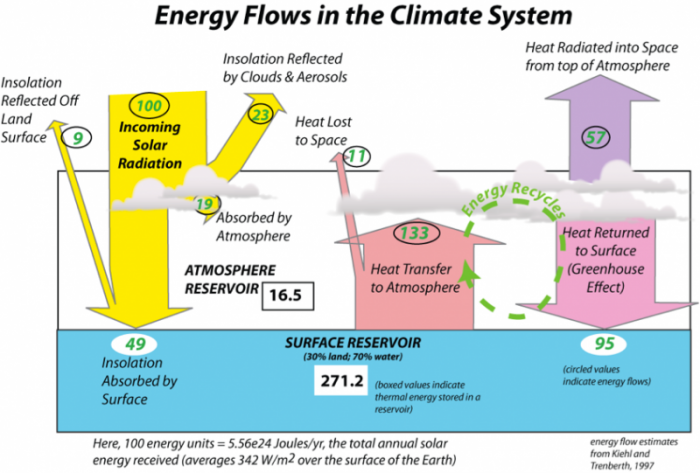
The image is a diagram titled "Energy Flows in the Climate System," illustrating the flow of solar energy through Earth's climate system, including the atmosphere and surface reservoirs. It quantifies the energy in units (relative to 100 units of incoming solar radiation) and highlights processes like reflection, absorption, heat transfer, and the greenhouse effect. The diagram is attributed to Kiehl and Trenberth (1997).
- Overall Structure:
- The diagram is divided into three main sections: incoming solar radiation (left), the atmosphere (center), and the surface reservoir (bottom). Arrows and numerical values indicate the flow and distribution of energy.
- Incoming Solar Radiation (Left Side):
- 100 units: Represented as a yellow arrow labeled "Incoming Solar Radiation," entering from the top left.
- 9 units: Reflected off the land surface, labeled "Insolation Reflected off Land Surface" (yellow arrow pointing back upward).
- 23 units: Reflected by clouds and aerosols, labeled "Insolation Reflected by Clouds & Aerosols" (yellow arrow pointing upward).
- Total Reflected: 9 + 23 = 32 units of the incoming 100 units are reflected back to space.
- Atmosphere (Center Section):
- 19 units: Absorbed by the atmosphere, labeled "Absorbed by Atmosphere" (yellow arrow curving into the atmosphere).
- 16.5 units: Labeled "Atmosphere Reservoir," indicating the energy stored in the atmosphere.
- 11 units: Lost to space as heat, labeled "Heat Lost to Space" (purple arrow pointing upward).
- 133 units: Transferred from the surface to the atmosphere, labeled "Heat Transfer to Atmosphere" (red arrow pointing upward).
- 57 units: Radiated into space from the top of the atmosphere, labeled "Heat Radiated into Space from top Atmosphere" (purple arrow pointing upward).
- 95 units: Returned to the surface via the greenhouse effect, labeled "Heat Returned to Surface (Greenhouse Effect)" (green arrow pointing downward).
- A section labeled "Energy Recycles" indicates the cycling of energy between the surface and atmosphere.
- Surface Reservoir (Bottom Section):
- 49 units: Absorbed by the surface, labeled "Insolation Absorbed by Surface" (yellow arrow pointing downward).
- 271.2 units: Labeled "Surface Reservoir (30% land; 70% water)," indicating the total energy at the surface.
- A note within the surface reservoir states: "(boxed values indicate thermal energy stored in a reservoir)," referring to the 271.2 units.
- Another note states: "(circled values indicate energy flows)," referring to the other numerical values in the diagram.
- Energy Balance Note (Bottom Left):
- A note at the bottom left reads: "Here, 100 energy units = ~5.56E24 Joules/yr; the total annual solar energy received (averages ~342 W/m² over the surface of the Earth)," providing the scale for the energy units used in the diagram.
- Visual Elements:
- The diagram uses color-coded arrows to represent different energy flows:
- Yellow for incoming and reflected solar radiation.
- Red for heat transfer from the surface to the atmosphere.
- Purple for heat lost to space.
- Green for heat returned to the surface via the greenhouse effect.
- Clouds are depicted in the atmosphere, and the surface is divided into land (30%) and water (70%).
- The diagram uses color-coded arrows to represent different energy flows:
The diagram effectively illustrates the energy balance of Earth's climate system, showing how incoming solar radiation is distributed, reflected, absorbed, and re-radiated, with a significant portion recycled through the greenhouse effect, as quantified by Kiehl and Trenberth in 1997.
When the insolation strikes the atmosphere, 23 units are reflected back to space from clouds and aerosols, which are tiny particles suspended in the atmosphere. Another 19 units are absorbed by the atmosphere, as described in the figure above, thus adding thermal energy to the atmosphere. The remaining 58 units of energy reach the Earth’s surface, where 9 units are reflected back into space, and the remaining 49 units are absorbed by the surface, warming the planet. The Earth’s surface is mostly water, and by virtue of its temperature and heat capacity, it has a lot more thermal energy than the atmosphere (271.2 vs 16.5). Energy flows up from the surface to the atmosphere in a variety of ways — mainly by emission of infrared radiation, heat transfer by evaporation, and then condensation of water. When water evaporates, it “steals” energy from the surface; this energy is needed to make the phase change from liquid to vapor, and the same energy is then released when water vapor condenses to form liquid water droplets. As you can see from the diagram, the combined flow of energy from the surface is greater than the amount we get from the sun! Of this energy given off by the surface, a little bit (7 units) escapes the atmosphere because there are no gases that absorb infrared energy at wavelengths between 10 to 15 microns; the rest is absorbed by the atmosphere, which then emits infrared energy from its top to outer space and from its bottom back to the surface; this atmospheric absorption of infrared energy and its return to the surface is called the greenhouse effect. Since the bottom of the atmosphere is much warmer than the top, much more energy is returned to the Earth’s surface than is emitted to outer space.
The remarkable thing to observe and remember here is that the surface receives almost twice as much energy from the greenhouse effect than it does directly from the Sun! But, if you look at the diagram a bit, you can see that the energy sent to the surface from the atmosphere is essentially recycled energy, whose origin is the Sun.
