Electromagnetic Spectrum
Brief Review of Electromagnetic Radiation
The energy we are concerned with here comes in the form of electromagnetic radiation, so it will help us to review some aspects of this form of energy. Electromagnetic (EM) radiation comes in a spectrum of waves, each consisting of an electrical and a magnetic oscillation of particles called photons; this spectrum is shown in the figure below:
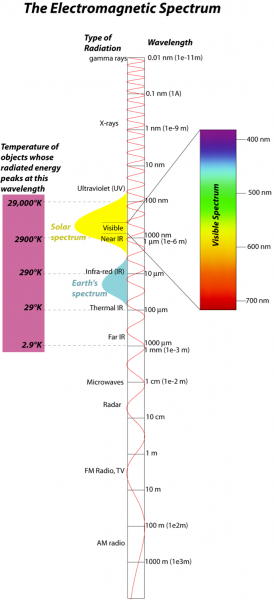
The image is a diagram titled "The Electromagnetic Spectrum," illustrating the range of electromagnetic radiation types, their wavelengths, and their relationship to the temperature of objects emitting them. The diagram is structured vertically, with various segments representing different types of radiation, their wavelengths, and associated temperatures.
- Title: "The Electromagnetic Spectrum" is written at the top.
- Main Structure: The diagram is a vertical bar divided into segments, each representing a type of electromagnetic radiation, with wavelengths and temperatures labeled on the sides.
- Wavelength Scale (Right Side):
- Wavelengths are shown in a logarithmic scale, ranging from 0.01 nm (nanometers) at the top to 1000 m (meters) at the bottom.
- Specific wavelength ranges are marked:
- 0.01 nm (10-11 m) for gamma rays
- 1 nm (10-9 m) for X-rays
- 10 nm for ultraviolet (UV)
- 100 nm to 1000 nm (10-6 m) for visible light (400 nm to 700 nm highlighted in a color gradient from purple to red)
- 10 μm (micrometers, 10-5 m) for infrared (IR)
- 100 μm for thermal IR
- 1000 μm (10-3 m) for far IR
- 1 cm (10-2 m) for microwaves
- 10 cm for radar
- 1 m for FM radio and TV
- 10 m to 1000 m (103 m) for AM radio
- Types of Radiation (Center):
- From top to bottom, the types of electromagnetic radiation are labeled:
- Gamma rays
- X-rays
- Ultraviolet (UV)
- Visible light (with a color gradient from purple at 400 nm to red at 700 nm)
- Near IR (infrared)
- Thermal IR
- Far IR
- Microwaves
- Radar
- FM radio, TV
- AM radio
- From top to bottom, the types of electromagnetic radiation are labeled:
- Temperature Scale (Left Side):
- The left side shows the temperature of objects whose energy peaks at specific wavelengths, based on blackbody radiation principles.
- Temperatures are marked with corresponding radiation types:
- 29,000°K (Kelvin) for objects emitting gamma rays
- 290°K for objects emitting in the solar spectrum (visible light)
- 29°K for objects emitting in the Earth's thermal IR spectrum
- 2.9°K for objects emitting in the microwave spectrum
- Spectral Curves:
- Two curves are overlaid on the diagram, showing the blackbody radiation spectra:
- A yellow curve labeled "Solar" peaks around the visible light range (290°K), indicating the Sun's emission spectrum.
- A blue curve labeled "Earth's" peaks in the thermal IR range (29°K), indicating Earth's emission spectrum.
- Two curves are overlaid on the diagram, showing the blackbody radiation spectra:
- Visible Light Section:
- The visible light portion (400 nm to 700 nm) is highlighted with a color gradient, transitioning from purple (400 nm) to blue, green, yellow, orange, and red (700 nm).
The diagram effectively illustrates the relationship between wavelength, type of electromagnetic radiation, and the temperature of objects emitting that radiation, emphasizing the Sun's peak in visible light and Earth's peak in thermal infrared.
